Molecular Basis of C-N Bond Cleavage by the Glycyl Radical Enzyme Choline Trimethylamine-Lyase
- PMID: 27642068
- PMCID: PMC5493019
- DOI: 10.1016/j.chembiol.2016.07.020
Molecular Basis of C-N Bond Cleavage by the Glycyl Radical Enzyme Choline Trimethylamine-Lyase
Abstract
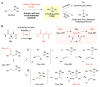
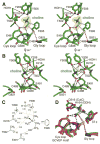
Deamination of choline catalyzed by the glycyl radical enzyme choline trimethylamine-lyase (CutC) has emerged as an important route for the production of trimethylamine, a microbial metabolite associated with both human disease and biological methane production. Here, we have determined five high-resolution X-ray structures of wild-type CutC and mechanistically informative mutants in the presence of choline. Within an unexpectedly polar active site, CutC orients choline through hydrogen bonding with a putative general base, and through close interactions between phenolic and carboxylate oxygen atoms of the protein scaffold and the polarized methyl groups of the trimethylammonium moiety. These structural data, along with biochemical analysis of active site mutants, support a mechanism that involves direct elimination of trimethylamine. This work broadens our understanding of radical-based enzyme catalysis and will aid in the rational design of inhibitors of bacterial trimethylamine production.
Copyright © 2016 Elsevier Ltd. All rights reserved.
Figures


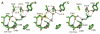

Comment in
-
Cutting Choline with Radical Scissors.Cell Chem Biol. 2016 Oct 20;23(10):1173-1174. doi: 10.1016/j.chembiol.2016.10.002. Cell Chem Biol. 2016. PMID: 27768865 Free PMC article.
Similar articles
-
Characterization of choline trimethylamine-lyase expands the chemistry of glycyl radical enzymes.ACS Chem Biol. 2014 Jul 18;9(7):1408-13. doi: 10.1021/cb500113p. Epub 2014 Jun 2. ACS Chem Biol. 2014. PMID: 24854437
-
Purification and Characterization of the Choline Trimethylamine-Lyase (CutC)-Activating Protein CutD.Methods Enzymol. 2018;606:73-94. doi: 10.1016/bs.mie.2018.04.012. Epub 2018 Jun 1. Methods Enzymol. 2018. PMID: 30097105
-
Structure and Function of CutC Choline Lyase from Human Microbiota Bacterium Klebsiella pneumoniae.J Biol Chem. 2015 Aug 28;290(35):21732-40. doi: 10.1074/jbc.M115.670471. Epub 2015 Jul 17. J Biol Chem. 2015. PMID: 26187464 Free PMC article.
-
New tricks for the glycyl radical enzyme family.Crit Rev Biochem Mol Biol. 2017 Dec;52(6):674-695. doi: 10.1080/10409238.2017.1373741. Epub 2017 Sep 13. Crit Rev Biochem Mol Biol. 2017. PMID: 28901199 Free PMC article. Review.
-
Discovering radical-dependent enzymes in the human gut microbiota.Curr Opin Chem Biol. 2018 Dec;47:86-93. doi: 10.1016/j.cbpa.2018.09.011. Epub 2018 Sep 27. Curr Opin Chem Biol. 2018. PMID: 30268905 Review.
Cited by
-
Modulation of the Gut Microbiota by Resistant Starch as a Treatment of Chronic Kidney Diseases: Evidence of Efficacy and Mechanistic Insights.Adv Nutr. 2019 Mar 1;10(2):303-320. doi: 10.1093/advances/nmy068. Adv Nutr. 2019. PMID: 30668615 Free PMC article. Review.
-
The gene expression and bioinformatic analysis of choline trimethylamine-lyase (CutC) and its activating enzyme (CutD) for gut microbes and comparison with their TMA production levels.Curr Res Microb Sci. 2021 Jun 17;2:100043. doi: 10.1016/j.crmicr.2021.100043. eCollection 2021 Dec. Curr Res Microb Sci. 2021. PMID: 34841334 Free PMC article.
-
Elucidation of an anaerobic pathway for metabolism of l-carnitine-derived γ-butyrobetaine to trimethylamine in human gut bacteria.Proc Natl Acad Sci U S A. 2021 Aug 10;118(32):e2101498118. doi: 10.1073/pnas.2101498118. Proc Natl Acad Sci U S A. 2021. PMID: 34362844 Free PMC article.
-
Insight into the Mechanism of Phenylacetate Decarboxylase (PhdB), a Toluene-Producing Glycyl Radical Enzyme.Chembiochem. 2020 Mar 2;21(5):663-671. doi: 10.1002/cbic.201900560. Epub 2019 Nov 7. Chembiochem. 2020. PMID: 31512343 Free PMC article.
-
Structural basis of carnitine monooxygenase CntA substrate specificity, inhibition, and intersubunit electron transfer.J Biol Chem. 2021 Jan-Jun;296:100038. doi: 10.1074/jbc.RA120.016019. Epub 2020 Nov 23. J Biol Chem. 2021. PMID: 33158989 Free PMC article.
References
-
- Adams PD, Afonine PV, Bunkoczi G, Chen VB, Davis IW, Echols N, Headd JJ, Hung LW, Kapral GJ, Grosse-Kunstleve RW, McCoy AJ, Moriarty NW, Oeffner R, Read RJ, Richardson DC, Richardson JS, Terwilliger TC, Zwart PH. PHENIX: a comprehensive Python-based system for macromolecular structure solution. Acta Crystallogr Sect D Biol Crystallogr. 2010;66:213–21. - PMC - PubMed
-
- Adhikari U, Scheiner S. Magnitude and mechanism of charge enhancement of CH··O hydrogen bonds. J Phys Chem A. 2013;117:10551–62. - PubMed
-
- Buckel W, Golding BT. Radical species in the catalytic pathways of enzymes from anaerobes. FEMS Microbiol Rev. 1998;22:523–541.
-
- Christodoulou J. Trimethylaminuria: an under-recognised and socially debilitating metabolic disorder. J Paediatr Child Health. 2012;48:E153–5. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

