Synthesis and Reactivity of Four- and Five-Coordinate Low-Spin Cobalt(II) PCP Pincer Complexes and Some Nickel(II) Analogues
- PMID: 27642210
- PMCID: PMC5021385
- DOI: 10.1021/om5007769
Synthesis and Reactivity of Four- and Five-Coordinate Low-Spin Cobalt(II) PCP Pincer Complexes and Some Nickel(II) Analogues
Abstract
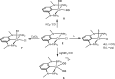
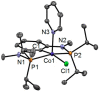
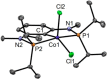
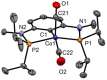
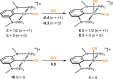
Anhydrous CoCl2 or [NiCl2(DME)] reacts with the ligand PCPMe-iPr (1) in the presence of nBuLi to afford the 15e and 16e square planar complexes [Co(PCPMe-iPr)Cl] (2) and [Ni(PCPMe-iPr)Cl] (3), respectively. Complex 2 is a paramagnetic d7 low-spin complex, which is a useful precursor for a series of Co(I), Co(II), and Co(III) PCP complexes. Complex 2 reacts readily with CO and pyridine to afford the five-coordinate square-pyramidal 17e complexes [Co(PCPMe-iPr)(CO)Cl] (4) and [Co(PCPMe-iPr)(py)Cl] (5), respectively, while in the presence of Ag+ and CO the cationic complex [Co(PCPMe-iPr)(CO)2]+ (6) is afforded. The effective magnetic moments μeff of all Co(II) complexes were derived from the temperature dependence of the inverse molar magnetic susceptibility by SQUID measurements and are in the range 1.9 to 2.4 μB. This is consistent with a d7 low-spin configuration with some degree of spin-orbit coupling. Oxidation of 2 with CuCl2 affords the paramagnetic Co(III) PCP complex [Co(PCPMe-iPr)Cl2] (7), while the synthesis of the diamagnetic Co(I) complex [Co(PCPMe-iPr)(CO)2] (8) was achieved by stirring 2 in toluene with KC8 in the presence of CO. Finally, the cationic 16e Ni(II) PCP complex [Ni(PCPMe-iPr)(CO)]+ (10) was obtained by reacting complex 3 with 1 equiv of AgSbF6 in the presence of CO. The reactivity of CO addition to Co(I), Co(II), and Ni(II) PCP square planar complexes of the type [M(PCPMe-iPr)(CO)] n (n = +1, 0) was investigated by DFT calculations, showing that formation of the Co species, 6 and 8, is thermodynamically favorable, while Ni(II) maintains the 16e configuration since CO addition is unfavorable in this case. X-ray structures of most complexes are provided and discussed. A structural feature of interest is that the apical CO ligand in 4 deviates significantly from linearity, with a Co-C-O angle of 170.0(1)°. The DFT-calculated value is 172°, clearly showing that this is not a packing but an electronic effect.
Conflict of interest statement
The authors declare no competing financial interest.
Figures

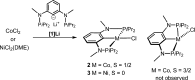






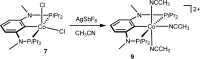
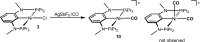

Similar articles
-
Synthesis, Characterization, and Catalytic Reactivity of {CoNO}8 PCP Pincer Complexes.Organometallics. 2020 Jul 27;39(14):2594-2601. doi: 10.1021/acs.organomet.0c00167. Epub 2020 Apr 24. Organometallics. 2020. PMID: 32742055 Free PMC article.
-
Synthesis, Structure, and Reactivity of Co(II) and Ni(II) PCP Pincer Borohydride Complexes.Organometallics. 2015 Apr 13;34(7):1364-1372. doi: 10.1021/acs.organomet.5b00075. Epub 2015 Mar 16. Organometallics. 2015. PMID: 27642212 Free PMC article.
-
Synthesis and reactivity of coordinatively unsaturated halocarbonyl molybdenum PNP pincer complexes.Dalton Trans. 2014 Oct 21;43(39):14669-79. doi: 10.1039/c4dt01932f. Dalton Trans. 2014. PMID: 25142749
-
Base metal complexes featuring a new pyrazole-derived PCP pincer ligand.Dalton Trans. 2023 Sep 13;52(35):12410-12422. doi: 10.1039/d3dt02111d. Dalton Trans. 2023. PMID: 37594380
-
Synthesis and reactivity of TADDOL-based chiral Fe(II) PNP pincer complexes-solution equilibria between κ(2)P,N- and κ(3)P,N,P-bound PNP pincer ligands.Dalton Trans. 2015 Aug 7;44(29):13071-86. doi: 10.1039/c5dt00832h. Dalton Trans. 2015. PMID: 26104487
Cited by
-
Synthesis, Characterization, and Catalytic Reactivity of {CoNO}8 PCP Pincer Complexes.Organometallics. 2020 Jul 27;39(14):2594-2601. doi: 10.1021/acs.organomet.0c00167. Epub 2020 Apr 24. Organometallics. 2020. PMID: 32742055 Free PMC article.
-
Synthesis and characterization of cobalt SCS pincer complexes.Monatsh Chem. 2022;153(7-8):545-549. doi: 10.1007/s00706-022-02949-1. Epub 2022 Jul 16. Monatsh Chem. 2022. PMID: 35966235 Free PMC article.
-
Solvothermal synthesis of cobalt PCP pincer complexes from [Co2(CO)8].Monatsh Chem. 2023;154(11):1253-1262. doi: 10.1007/s00706-023-03123-x. Epub 2023 Sep 9. Monatsh Chem. 2023. PMID: 37927400 Free PMC article.
-
Crystal structure of bis-[μ-2-(diiso-propyl-phosphor-yl)propan-2-olato-κ(3) O (1),O (2):O (1)]bis-[chlorido-oxidovanadium(IV)].Acta Crystallogr E Crystallogr Commun. 2016 May 6;72(Pt 6):785-8. doi: 10.1107/S2056989016007362. eCollection 2016 Jun 1. Acta Crystallogr E Crystallogr Commun. 2016. PMID: 27308042 Free PMC article.
-
Synthesis and Characterization of Cobalt NCN Pincer Complexes.Eur J Inorg Chem. 2021 Nov 8;2021(41):4280-4285. doi: 10.1002/ejic.202100643. Epub 2021 Oct 11. Eur J Inorg Chem. 2021. PMID: 34819799 Free PMC article.
References
-
-
For reviews on pincer complexes, see:
- Albrecht M.; van Koten G. Angew. Chem., Int. Ed. 2001, 40, 3750–3781. - PubMed
- van der Boom M. E.; Milstein D. Chem. Rev. 2003, 103, 1759–1792. - PubMed
- Singleton J. T. Tetrahedron 2003, 59, 1837.
- Bhattacharya P.; Guan H. Comment Inorg. Chem. 2011, 32, 88.
- Schneider S.; Meiners J.; Askevold B. Eur. J. Inorg. Chem. 2012, 412–429.
- Morales-Morales D.; Jensen C. M., Eds. The Chemistry of Pincer Compounds; Elsevier: Amsterdam, 2007.
- van Koten G., Milstein D., Eds.; Organometallic Pincer Chemistry; Topics in Organometallic Chemistry Vol. 40; Springer: Berlin, 2013.
-
-
- Benito-Garagorri D.; Kirchner K. Acc. Chem. Res. 2008, 41, 201–213. - PubMed
-
- Benito-Garagorri D.; Bocokić V.; Mereiter K.; Kirchner K. Organometallics 2006, 25, 3817–3823.
-
-
For recent examples of Fe PNP complexes based on the 2,6-diaminopyridine scaffold see:
- Bichler B.; Holzhacker C.; Stöger B.; Puchberger M.; Veiros L. F.; Kirchner K. Organometallics 2013, 32, 4114–4121. - PMC - PubMed
- Benito-Garagorri D.; Alves L. G.; Veiros L. F.; Standfest-Hauser C. M.; Tanaka S.; Mereiter K.; Kirchner K. Organometallics 2010, 29, 4932–4942.
- Benito-Garagorri D.; Puchberger M.; Mereiter K.; Kirchner K. Angew. Chem., Int. Ed. 2008, 47, 9142–9145. - PubMed
-
-
-
For recent examples of Mo PNP complexes based on the 2,6-diaminopyridine scaffold see:
- Öztopcu Ö.; Holzhacker C.; Puchberger M.; Weil M.; Mereiter K.; Veiros L. F.; Kirchner K. Organometallics 2013, 32, 3042–3052. - PMC - PubMed
- de Aguiar S. R. M. M.; Stöger B.; Pittenauer E.; Allmaier G.; Puchberger M.; Veiros L. F.; Kirchner K. J. Organomet. Chem. 2014, 760, 74–83. - PMC - PubMed
-
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
