The Mechanism of Long Non-coding RNA MEG3 for Neurons Apoptosis Caused by Hypoxia: Mediated by miR-181b-12/15-LOX Signaling Pathway
- PMID: 27642276
- PMCID: PMC5009716
- DOI: 10.3389/fncel.2016.00201
The Mechanism of Long Non-coding RNA MEG3 for Neurons Apoptosis Caused by Hypoxia: Mediated by miR-181b-12/15-LOX Signaling Pathway
Abstract
Objective: lncRNAs are recently thought to play a significant role in cellular homeostasis during pathological process of diseases by competing inhibiting miRNA function. The aim of present study was to assess the function of long non-coding RNA (lncRNA) MEG3 and its functional interaction with microRNA-181b in cerebral ischemic infarct of mice and hypoxia-induced neurons apoptosis.
Methods: To address this question, we performed the experiments with in vivo middle cerebral artery occlusion (MCAO) mice model and in vitro oxygen-glucose deprivation (OGD)-cultured neuronal HT22 cell line. Relative expression of MEG3, miR-181b, and 12/15-LOX (lipoxygenase) mRNA was determined using quantitative RT-PCR. Western blot was used to evaluate 12/15-LOX protein expression. TUNEL assay was performed to assess cell apoptosis.
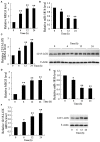
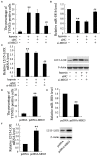
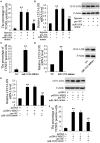
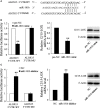
Results: In both MCAO mice and OGD-cultured HT22 cell, ischemia, or hypoxia treatment results in a time-dependent increase in MEG3 and 12/15-LOX expression and decrease in miR-181b expression. Knockdown of MEG3 contributes to attenuation of hypoxia-induced apoptosis of HT22 cell. Also, expression level of MEG3 negatively correlated with miR-181b expression and positively correlated with 12/15-LOX expression. In contrary to MEG3, miR-181b overexpression attenuated hypoxia-induced HT22 cell apoptosis, as well as suppressed hypoxia-induced increase in 12/15-LOX expression. By luciferase reporter assay, we concluded that miR-181b directly binds to 12/15-LOX 3'-UTR, thereby negatively regulates 12/15-LOX expression.
Conclusion: Our data suggested that long non-coding RNA MEG3 functions as a competing endogenous RNA for miR-181b to regulate 12/15-LOX expression in middle cerebral artery occlusion-induced ischemic infarct of brain nerve cells.
Keywords: 12/15-LOX; MCAO; MEG3; infarct; miR-181b.
Figures
Similar articles
-
Inhibition of lncRNA MEG3 protects renal tubular from hypoxia-induced kidney injury in acute renal allografts by regulating miR-181b/TNF-α signaling pathway.J Cell Biochem. 2019 Aug;120(8):12822-12831. doi: 10.1002/jcb.28553. Epub 2019 Mar 12. J Cell Biochem. 2019. PMID: 30860638
-
Long non-coding RNA MEG3 promotes cerebral ischemia-reperfusion injury through increasing pyroptosis by targeting miR-485/AIM2 axis.Exp Neurol. 2020 Mar;325:113139. doi: 10.1016/j.expneurol.2019.113139. Epub 2019 Nov 30. Exp Neurol. 2020. PMID: 31794744
-
The Long Non-coding RNA MEG3/miR-let-7c-5p Axis Regulates Ethanol-Induced Hepatic Steatosis and Apoptosis by Targeting NLRC5.Front Pharmacol. 2018 Apr 10;9:302. doi: 10.3389/fphar.2018.00302. eCollection 2018. Front Pharmacol. 2018. PMID: 29692724 Free PMC article.
-
Long non-coding RNA MEG3 functions as a competing endogenous RNA to regulate ischemic neuronal death by targeting miR-21/PDCD4 signaling pathway.Cell Death Dis. 2017 Dec 13;8(12):3211. doi: 10.1038/s41419-017-0047-y. Cell Death Dis. 2017. PMID: 29238035 Free PMC article.
-
The multifaceted biology of lncR-Meg3 in cardio-cerebrovascular diseases.Front Genet. 2023 Mar 10;14:1132884. doi: 10.3389/fgene.2023.1132884. eCollection 2023. Front Genet. 2023. PMID: 36968595 Free PMC article. Review.
Cited by
-
MEG3 lncRNA is over-expressed in autism spectrum disorder.Metab Brain Dis. 2021 Dec;36(8):2235-2242. doi: 10.1007/s11011-021-00764-x. Epub 2021 Jun 11. Metab Brain Dis. 2021. PMID: 34115273
-
Pathogenesis of Ischemic Stroke: Role of Epigenetic Mechanisms.Genes (Basel). 2020 Jan 13;11(1):89. doi: 10.3390/genes11010089. Genes (Basel). 2020. PMID: 31941075 Free PMC article. Review.
-
Comprehensive Analysis of Hub Genes Associated With Competing Endogenous RNA Networks in Stroke Using Bioinformatics Analysis.Front Genet. 2022 Jan 12;12:779923. doi: 10.3389/fgene.2021.779923. eCollection 2021. Front Genet. 2022. PMID: 35096003 Free PMC article.
-
LncRNA GACAT1 induces tongue squamous cell carcinoma migration and proliferation via miR-149.J Cell Mol Med. 2021 Sep;25(17):8215-8221. doi: 10.1111/jcmm.16690. Epub 2021 Aug 10. J Cell Mol Med. 2021. PMID: 34378327 Free PMC article.
-
Association between MEG3/miR-181b polymorphisms and risk of ischemic stroke.Lipids Health Dis. 2018 Dec 22;17(1):292. doi: 10.1186/s12944-018-0941-z. Lipids Health Dis. 2018. PMID: 30579356 Free PMC article.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

