In-Vitro Characterization and Oral Bioavailability of Organic Solvent-free Solid Dispersions Containing Telmisartan
- PMID: 27642309
- PMCID: PMC5018266
In-Vitro Characterization and Oral Bioavailability of Organic Solvent-free Solid Dispersions Containing Telmisartan
Abstract
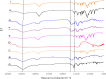
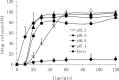
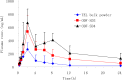
Poorly water-soluble drugs often suffer from limited or irreproducible clinical response due to their low solubility and dissolution rate. In this study, organic solvent-free solid dispersions (OSF-SDs) containing telmisartan (TEL) were prepared using polyvinylpyrrolidone K30 (PVP K30) and polyethylene glycol 6000 (PEG 6000) as hydrophilic polymers, sodium hydroxide (NaOH) as an alkalizer, and poloxamer 188 as a surfactant by a lyophilization method. In-vitro dissolution rate and physicochemical properties of the OSF-SDs were characterized using the USP I basket method, differential scanning calorimetry (DSC), X-ray diffractometry (XRD) and fourier transform-infrared (FT-IR) spectroscopy. In addition, the oral bioavailability of OSF-SDs in rats was evaluated by using TEL bulk powder as a reference. The dissolution rates of the OSF-SDs were significantly enhanced as compared to TEL bulk powder. The results from DSC, XRD showed that TEL was molecularly dispersed in the OSF-SDs as an amorphous form. The FT-IR results suggested that intermolecular hydrogen bonding had formed between TEL and its carriers. The OSF-SDs exhibited significantly higher AUC0-24 h and Cmax, but similar Tmax as compared to the reference. This study demonstrated that OSF-SDs can be a promising method to enhance the dissolution rate and oral bioavailability of TEL.
Keywords: In-vitro characterization; Oral bioavailability; Organic Solvent-free; Solid dispersions; Telmisartan.
Figures




Similar articles
-
Lyophilized Amorphous Dispersion of Telmisartan in a Combined Carrier-Alkalizer System: Formulation Development and In Vivo Study.ACS Omega. 2020 Dec 10;5(50):32466-32480. doi: 10.1021/acsomega.0c04588. eCollection 2020 Dec 22. ACS Omega. 2020. PMID: 33376884 Free PMC article.
-
Enhanced dissolution and oral bioavailability of valsartan solid dispersions prepared by a freeze-drying technique using hydrophilic polymers.Drug Deliv. 2016;23(1):41-8. doi: 10.3109/10717544.2014.903012. Epub 2015 Sep 4. Drug Deliv. 2016. PMID: 24735247
-
Stability and Bioavailability Enhancement of Telmisartan Ternary Solid Dispersions: the Synergistic Effect of Polymers and Drug-Polymer(s) Interactions.AAPS PharmSciTech. 2019 Mar 18;20(4):143. doi: 10.1208/s12249-019-1358-3. AAPS PharmSciTech. 2019. PMID: 30887265
-
In vitro dissolution and physicochemical characterizations of novel PVP-based solid dispersions containing valsartan prepared by a freeze-drying method.Pak J Pharm Sci. 2014 Nov;27(6):1799-804. Pak J Pharm Sci. 2014. PMID: 25362604
-
Physicochemical characterization and dissolution study of solid dispersions of Lovastatin with polyethylene glycol 4000 and polyvinylpyrrolidone K30.Pharm Dev Technol. 2007;12(1):21-33. doi: 10.1080/10837450601166510. Pharm Dev Technol. 2007. PMID: 17484141
Cited by
-
Critical Analysis and Optimization of Stoichiometric Ratio of Drug-Coformer on Cocrystal Design: Molecular Docking, In Vitro and In Vivo Assessment.Pharmaceuticals (Basel). 2023 Feb 13;16(2):284. doi: 10.3390/ph16020284. Pharmaceuticals (Basel). 2023. PMID: 37259428 Free PMC article.
-
Transdermal Delivery of Telmisartan: Formulation, in vitro, ex vivo, Iontophoretic Permeation Enhancement and Comparative Pharmacokinetic Study in Rats.Drug Des Devel Ther. 2021 Nov 10;15:4603-4614. doi: 10.2147/DDDT.S327860. eCollection 2021. Drug Des Devel Ther. 2021. PMID: 34785889 Free PMC article.
-
Effect of Process Parameters on Nano-Microparticle Formation During Supercritical Antisolvent Process Using Mixed Solvent: Application for Enhanced Dissolution and Oral Bioavailability of Telmisartan Through Particle-Size Control Based on Experimental Design.Pharmaceutics. 2024 Nov 24;16(12):1508. doi: 10.3390/pharmaceutics16121508. Pharmaceutics. 2024. PMID: 39771489 Free PMC article.
-
Lyophilized Amorphous Dispersion of Telmisartan in a Combined Carrier-Alkalizer System: Formulation Development and In Vivo Study.ACS Omega. 2020 Dec 10;5(50):32466-32480. doi: 10.1021/acsomega.0c04588. eCollection 2020 Dec 22. ACS Omega. 2020. PMID: 33376884 Free PMC article.
-
Doxorubicin and anti-VEGF siRNA co-delivery via nano-graphene oxide for enhanced cancer therapy in vitro and in vivo.Int J Nanomedicine. 2018 Jun 27;13:3713-3728. doi: 10.2147/IJN.S162939. eCollection 2018. Int J Nanomedicine. 2018. PMID: 29983564 Free PMC article.
References
-
- Wienen W, Entzeroth M, van Meel JCA, Stangier J, Busch U, Ebner T, Schmid J, Lehmann H, Matzek K, Kempthorne-Rawson J, Gladigau V, Hauel NH. A review on telmisartan: A novel, long-acting angiotensin II-receptor antagonist. Cardiovasc Drug Rev. 2000;18:127–154.
-
- Tran PHL, Tran HTT, Lee BJ. Modulation of microenvironmental pH and crystallinity of ionizable TEL using alkalizers in solid dispersions for controlled release. J Control Rel. 2008;129:59–65. - PubMed
-
- Van den Mooter G, Augustijns P, Blaton N, Kinget R. Physico-chemical characterization of solid dispersions of temazepam with polyethylene glycol 6000 and PVP K30. Int J Pharm. 1998;164:67–80.
LinkOut - more resources
Full Text Sources
