Toxicological assessment of the hydroethanolic leaf extract of clerodendrum capitatum in Wistar rats
- PMID: 27642406
- PMCID: PMC5012807
- DOI: 10.11604/pamj.2016.24.66.8771
Toxicological assessment of the hydroethanolic leaf extract of clerodendrum capitatum in Wistar rats
Abstract
Introduction: Clerodendrum capitatum (Willd) Schumach. & Thonn (Lamiaceae) is used in African traditional medicine for the treatment of malaria, hypertension, obesity, jaundice and diabetes however there is lack of experimental data on its possible toxicity. This study investigated the acute and 28 days sub-chronic toxicity of C. capitatum in Wistar rats.
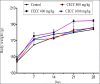
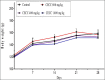
Methods: In acute toxicity tests, a single administration of the hydroethanolic C. capitatum leaf extract (5 g/kg) was given orally to 5 female rats. The general behavior, adverse effects and mortality were recorded for up to 14 days post treatment. On the 15(th) day, the rats were weighed and euthanized for necropsy. In sub-chronic toxicity tests, the extract (4, 8 and 16 g/kg/day) was given orally to both male and female rats for 28 days. The animal body weight was recorded throughout the experiment, while hematological and biochemical parameters of blood and relative organs weights were evaluated on the 29(th) day.
Results: Clerodendrum Capitatum did not cause any death or any hazardous symptoms of acute toxicity, showing an LD50 higher than 5 g/kg. Sub-chronic administration of C. capitatum resulted in no noticeable changes in weight gain and water or food consumption. White blood cells and hemoglobin increased while urea concentration, liver enzymes, total cholesterol and glucose concentrations significantly decreased in treated animals. No changes in macroscopical aspect of organs were observed in the animals.
Conclusion: These results showed that acute or sub-chronic oral administration of the hydroethanolic leaf extract of Clerodendrum capitatum may be considered as relatively free of toxicity.
Keywords: Clerodendrum capitatum; Wistar rats; oral toxicity; safety.
Figures
References
-
- Bennett J, Brown CM. Use of herbal remedies by patients in a health maintenance organization. J Am Pharm Assoc (Wash) 2000 May-Jun;40(3):353–8. - PubMed
-
- Kirby GC. Plants as sources of antimalarial components. Trop Doct. 1997;27(1):7–11. - PubMed
-
- Warrell DA. Herbal remedies for malaria. Trop Doct. 1997 Jan;27(1):5–6. - PubMed
-
- Elisabetsky E, Amadar TA, Albuquerque RR, Nunes DS, Carvalho ADC. Analgesic activity of Psychotria colorata (Willd Exr & S) Muell. Arg alkaloid J Ethnopharmacol. 1995 Oct;48(2):77–83. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources