Lack of immunoediting in murine pancreatic cancer reversed with neoantigen
- PMID: 27642636
- PMCID: PMC5026128
- DOI: 10.1172/jci.insight.88328
Lack of immunoediting in murine pancreatic cancer reversed with neoantigen
Abstract
In carcinogen-driven cancers, a high mutational burden results in neoepitopes that can be recognized immunologically. Such carcinogen-induced tumors may evade this immune response through "immunoediting," whereby tumors adapt to immune pressure and escape T cell-mediated killing. Many tumors lack a high neoepitope burden, and it remains unclear whether immunoediting occurs in such cases. Here, we evaluated T cell immunity in an autochthonous mouse model of pancreatic cancer and found a low mutational burden, absence of predicted neoepitopes derived from tumor mutations, and resistance to checkpoint immunotherapy. Spontaneous tumor progression was identical in the presence or absence of T cells. Moreover, tumors arising in T cell-depleted mice grew unchecked in immune-competent hosts. However, introduction of the neoantigen ovalbumin (OVA) led to tumor rejection and T cell memory, but this did not occur in OVA immune-tolerant mice. Thus, immunoediting does not occur in this mouse model - a likely consequence, not a cause, of absent neoepitopes. Because many human tumors also have a low missense mutational load and minimal neoepitope burden, our findings have clinical implications for the design of immunotherapy for patients with such tumors.
Conflict of interest statement
The authors have declared that no conflict of interest exists.
Figures
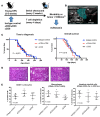
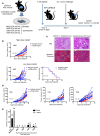
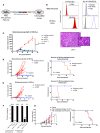


References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

