Costimulatory Effects of an Immunodominant Parasite Antigen Paradoxically Prevent Induction of Optimal CD8 T Cell Protective Immunity
- PMID: 27642757
- PMCID: PMC5028030
- DOI: 10.1371/journal.ppat.1005896
Costimulatory Effects of an Immunodominant Parasite Antigen Paradoxically Prevent Induction of Optimal CD8 T Cell Protective Immunity
Abstract
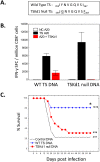
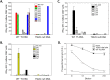
Trypanosoma cruzi infection is controlled but not eliminated by host immunity. The T. cruzi trans-sialidase (TS) gene superfamily encodes immunodominant protective antigens, but expression of altered peptide ligands by different TS genes has been hypothesized to promote immunoevasion. We molecularly defined TS epitopes to determine their importance for protection versus parasite persistence. Peptide-pulsed dendritic cell vaccination experiments demonstrated that one pair of immunodominant CD4+ and CD8+ TS peptides alone can induce protective immunity (100% survival post-lethal parasite challenge). TS DNA vaccines have been shown by us (and others) to protect BALB/c mice against T. cruzi challenge. We generated a new TS vaccine in which the immunodominant TS CD8+ epitope MHC anchoring positions were mutated, rendering the mutant TS vaccine incapable of inducing immunity to the immunodominant CD8 epitope. Immunization of mice with wild type (WT) and mutant TS vaccines demonstrated that vaccines encoding enzymatically active protein and the immunodominant CD8+ T cell epitope enhance subdominant pathogen-specific CD8+ T cell responses. More specifically, CD8+ T cells from WT TS DNA vaccinated mice were responsive to 14 predicted CD8+ TS epitopes, while T cells from mutant TS DNA vaccinated mice were responsive to just one of these 14 predicted TS epitopes. Molecular and structural biology studies revealed that this novel costimulatory mechanism involves CD45 signaling triggered by enzymatically active TS. This enhancing effect on subdominant T cells negatively regulates protective immunity. Using peptide-pulsed DC vaccination experiments, we have shown that vaccines inducing both immunodominant and subdominant epitope responses were significantly less protective than vaccines inducing only immunodominant-specific responses. These results have important implications for T. cruzi vaccine development. Of broader significance, we demonstrate that increasing breadth of T cell epitope responses induced by vaccination is not always advantageous for host immunity.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures





Similar articles
-
DNA sequences encoding CD4+ and CD8+ T-cell epitopes are important for efficient protective immunity induced by DNA vaccination with a Trypanosoma cruzi gene.Infect Immun. 2001 Sep;69(9):5477-86. doi: 10.1128/IAI.69.9.5477-5486.2001. Infect Immun. 2001. PMID: 11500420 Free PMC article.
-
Trans-sialidase recombinant protein mixed with CpG motif-containing oligodeoxynucleotide induces protective mucosal and systemic trypanosoma cruzi immunity involving CD8+ CTL and B cell-mediated cross-priming.J Immunol. 2007 Nov 15;179(10):6889-900. doi: 10.4049/jimmunol.179.10.6889. J Immunol. 2007. PMID: 17982080
-
An immunoinformatic approach for identification of Trypanosoma cruzi HLA-A2-restricted CD8(+) T cell epitopes.Hum Vaccin Immunother. 2015;11(9):2322-8. doi: 10.1080/21645515.2015.1061160. Epub 2015 Jun 24. Hum Vaccin Immunother. 2015. PMID: 26107442 Free PMC article.
-
CD8+ T cells in Trypanosoma cruzi infection.Semin Immunopathol. 2015 May;37(3):233-8. doi: 10.1007/s00281-015-0481-9. Epub 2015 Apr 29. Semin Immunopathol. 2015. PMID: 25921214 Free PMC article. Review.
-
CD8(+) T cell-mediated immunity during Trypanosoma cruzi infection: a path for vaccine development?Mediators Inflamm. 2014;2014:243786. doi: 10.1155/2014/243786. Epub 2014 Jul 1. Mediators Inflamm. 2014. PMID: 25104879 Free PMC article. Review.
Cited by
-
Novel Identified HLA-A*0201-Restricted Hantaan Virus Glycoprotein Cytotoxic T-Cell Epitopes Could Effectively Induce Protective Responses in HLA-A2.1/Kb Transgenic Mice May Associate with the Severity of Hemorrhagic Fever with Renal Syndrome.Front Immunol. 2017 Dec 12;8:1797. doi: 10.3389/fimmu.2017.01797. eCollection 2017. Front Immunol. 2017. PMID: 29312318 Free PMC article.
-
Recombinant Mycobacterium bovis BCG is a promising platform to develop vaccines against Trypansoma cruzi infection.Clin Exp Immunol. 2020 Sep;201(3):306-316. doi: 10.1111/cei.13469. Epub 2020 Jul 6. Clin Exp Immunol. 2020. PMID: 32464684 Free PMC article.
-
Trans-sialidase-based vaccine candidate protects against Trypanosoma cruzi infection, not only inducing an effector immune response but also affecting cells with regulatory/suppressor phenotype.Oncotarget. 2017 May 25;8(35):58003-58020. doi: 10.18632/oncotarget.18217. eCollection 2017 Aug 29. Oncotarget. 2017. PMID: 28938533 Free PMC article.
-
Use of Leishmania major parasites expressing a recombinant Trypanosoma cruzi antigen as live vaccines against Chagas disease.Front Microbiol. 2022 Nov 29;13:1059115. doi: 10.3389/fmicb.2022.1059115. eCollection 2022. Front Microbiol. 2022. PMID: 36523834 Free PMC article.
-
Control of myeloid-derived suppressor cell dynamics potentiates vaccine protection in multiple mouse models of Trypanosoma cruzi infection.Front Immunol. 2024 Nov 1;15:1484290. doi: 10.3389/fimmu.2024.1484290. eCollection 2024. Front Immunol. 2024. PMID: 39555082 Free PMC article.
References
-
- Hoft DF, Lynch RG, Kirchhoff LV. Kinetic analysis of antigen-specific immune responses in resistant and susceptible mice during infection with Trypanosoma cruzi . J Immunol. 1993;151(12):7038–47. . - PubMed
-
- Tarleton RL. Depletion of CD8+ T cells increases susceptibility and reverses vaccine-induced immunity in mice infected with Trypanosoma cruzi . J Immunol. 1990;144(2):717–24. . - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

