New Regio- and Stereoselective Cascades via Unstabilized Azomethine Ylide Cycloadditions for the Synthesis of Highly Substituted Tropane and Indolizidine Frameworks
- PMID: 27642766
- PMCID: PMC5066814
- DOI: 10.1021/jacs.6b08355
New Regio- and Stereoselective Cascades via Unstabilized Azomethine Ylide Cycloadditions for the Synthesis of Highly Substituted Tropane and Indolizidine Frameworks
Abstract
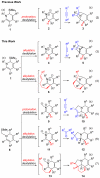
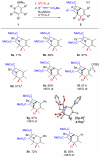
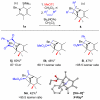
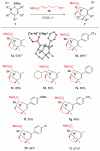
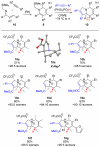
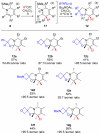
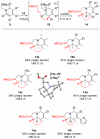
Multisubstituted tropanes and indolizidines have been prepared with high regio- and stereoselectivity by the [3+2] cycloaddition of unstabilized azomethine ylides generated from readily prepared trimethylsilyl-substituted 1,2-dihydropyridines via protonation or alkylation followed by desilylation. Starting from 1,2-dihydropyridines bearing a ring trimethylsilyl substituent at the 6-position, an intermolecular alkylation/desilylation provides endocyclic unstabilized ylides that successfully undergo cycloaddition with a range of symmetrical and unsymmetrical alkyne and alkene dipolarophiles to afford densely substituted tropanes incorporating quaternary carbons in good yields and with high regio- and stereoselectivity. Additionally, an intramolecular alkylation/desilylation/cycloaddition sequence provides convenient and rapid entry to bridged tricyclic tropane skeletons, allowing for five contiguous carbon stereocenters to be set in a single experimental operation and under mild conditions. Starting from 1,2-dihydropyridines with trimethylsilylmethyl groups on nitrogen, protonation followed by desilylation generates exocyclic unstabilized ylides that undergo cycloaddition with unsymmetrical alkynes to give indolizidines with good regio- and stereoselectivity. N-Trimethylsilylmethyl-1,2-dihydropyridines can also be alkylated and subsequently desilylated to give endocyclic unstabilized ylides that undergo intermolecular cycloadditions with carbonyl compounds to give bicyclic oxazolidine products in good overall yields. Moreover, an intramolecular alkylation/desilylation/cycloaddition sequence with the N-trimethylsilylmethyl-1,2-dihydropyridines affords tricyclic indolizidines that incorporate quaternary carbons and up to five stereocenters with good to excellent regio- and diastereoselectivity.
Figures
References
-
- Michael JP. Nat. Prod. Rep. 2008;25:139–165. - PubMed
- O'Hagan D. Nat. Prod. Rep. 2000;17:435–446. - PubMed
- Lounasmaa M, Tamminen T. The Tropane Alkaloids. In: Cordell GA, editor. The Alkaloids: Chemistry and Pharmacology. Vol. 44. Academic Press; San Diego, CA: 1997. pp. 1–114.
- O'Hagan D. Nat. Prod. Rep. 1997;14:637–651.
- Fodor G, Dharanipragada R. Nat. Prod. Rep. 1994;11:443–450. - PubMed
- Gellert E. J. Nat. Prod. 1982;45:50–73.
-
- Belanger G, Darsigny V, Dore M, Levesque F. Org. Lett. 2010;12:1396–1399. - PubMed
- Coldham I, Jana S, Watson L, Pilgram CD. Tetrahedron Lett. 2008;49:5408–5410.
- Pearson WH, Stoy P, Mi Y. J. Org. Chem. 2004;69:1919–1939. - PubMed
- Pandey G, Laha JK, Lakshmaiah G. Tetrahedron. 2002;58:3525–3534.
- Castulik J, Marek J, Mazal C. Tetrahedron. 2001;57:8339–8347.
- Pandey G, Sahoo AK, Bagul TD. Org. Lett. 2000;2:2299–2301. - PubMed
- Pandey G, Laha JK, Mohanakrishnan AK. Tetrahedron Lett. 1999;40:6065–6068.
- Pearson WH, Mi Y. Tetrahedron Lett. 1997;38:5441–5444.
- Pandey G, Lakshmaiah G, Ghatak A. Tetrahedron Lett. 1993;34:7301–7304.
- Pandey G, Lakshmaiah G. Tetrahedron Lett. 1993;34:4861–4864.
-
- Yamada R, Adachi Y, Yokoshima S, Fukuyama T. Angew. Chem. Int. Ed. 2016;55:6067–6070. For recent applications of azomethine ylide cycloadditions in complex natural products syntheses, see: - PubMed
- Pandey G, Burugu SK, Singh P. Org. Lett. 2016;18:1558–1561. - PubMed
- Haddad S, Boudriga S, Porzio F, Soldera A, Askri M, Knorr M, Rousselin Y, Kubicki MM, Golz C, Strohmann C. J. Org. Chem. 2015;80:9064–9075. - PubMed
- Belanger G, Boudreault J, Levesque F. Org. Lett. 2011;13:6204–6207. - PubMed
- Coldham I, Burrell AJM, Guerrand HDS, Oram N. Org. Lett. 2011;13:1267–1269. - PubMed
- Davoren J, Gray D, Harris A, Nason D, Xu W. Synlett. 2010:2490–2492.
- Peese KM, Gin DY. Chem. Eur. J. 2008;14:1654–1665. - PMC - PubMed
- Eckelbarger JD, Wilmot JT, Epperson MT, Thakur CS, Shum D, Antczak C, Tarassishin L, Djaballah H, Gin DY. Chem. Eur. J. 2008;14:4293–4306. - PMC - PubMed
- Coldham I, Burrell AJM, White LE, Adams H, Oram N. Angew. Chem. Int. Ed. 2007;46:6159–6162. - PubMed
- Su S, Porco JA., Jr. J. Am. Chem. Soc. 2007;129:7744–7745. - PubMed
- Eckelbarger JD, Wilmot JT, Gin DY. J. Am. Chem. Soc. 2006;128:10370–10371. - PMC - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

