Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow
- PMID: 27642788
- PMCID: PMC5357601
- DOI: 10.1038/ncb3408
Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow
Erratum in
-
Erratum: Induction of LIFR confers a dormancy phenotype in breast cancer cells disseminated to the bone marrow.Nat Cell Biol. 2016 Oct 27;18(11):1260. doi: 10.1038/ncb3433. Nat Cell Biol. 2016. PMID: 27784904
Abstract
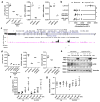
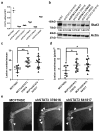
Breast cancer cells frequently home to the bone marrow, where they may enter a dormant state before forming a bone metastasis. Several members of the interleukin-6 (IL-6) cytokine family are implicated in breast cancer bone colonization, but the role for the IL-6 cytokine leukaemia inhibitory factor (LIF) in this process is unknown. We tested the hypothesis that LIF provides a pro-dormancy signal to breast cancer cells in the bone. In breast cancer patients, LIF receptor (LIFR) levels are lower with bone metastases and are significantly and inversely correlated with patient outcome and hypoxia gene activity. Hypoxia also reduces the LIFR:STAT3:SOCS3 signalling pathway in breast cancer cells. Loss of the LIFR or STAT3 enables otherwise dormant breast cancer cells to downregulate dormancy-, quiescence- and cancer stem cell-associated genes, and to proliferate in and specifically colonize the bone, suggesting that LIFR:STAT3 signalling confers a dormancy phenotype in breast cancer cells disseminated to bone.
Conflict of interest statement
Competing financial interests
The authors declare no competing financial interests.
Figures






Similar articles
-
gp130 Cytokines Activate Novel Signaling Pathways and Alter Bone Dissemination in ER+ Breast Cancer Cells.J Bone Miner Res. 2022 Feb;37(2):185-201. doi: 10.1002/jbmr.4430. Epub 2021 Sep 17. J Bone Miner Res. 2022. PMID: 34477239 Free PMC article.
-
HDAC inhibitors stimulate LIFR when it is repressed by hypoxia or PTHrP in breast cancer.J Bone Oncol. 2021 Dec 1;31:100407. doi: 10.1016/j.jbo.2021.100407. eCollection 2021 Dec. J Bone Oncol. 2021. PMID: 34934614 Free PMC article.
-
HDAC inhibitors induce LIFR expression and promote a dormancy phenotype in breast cancer.Oncogene. 2021 Aug;40(34):5314-5326. doi: 10.1038/s41388-021-01931-1. Epub 2021 Jul 10. Oncogene. 2021. PMID: 34247191 Free PMC article.
-
Targeting LIF/LIFR signaling in cancer.Genes Dis. 2021 Apr 29;9(4):973-980. doi: 10.1016/j.gendis.2021.04.003. eCollection 2022 Jul. Genes Dis. 2021. PMID: 35685476 Free PMC article. Review.
-
Emerging Perspectives on Leukemia Inhibitory Factor and its Receptor in Cancer.Front Oncol. 2021 Jul 29;11:693724. doi: 10.3389/fonc.2021.693724. eCollection 2021. Front Oncol. 2021. PMID: 34395259 Free PMC article. Review.
Cited by
-
Innovative Approaches in the Battle Against Cancer Recurrence: Novel Strategies to Combat Dormant Disseminated Tumor Cells.Front Oncol. 2021 Apr 27;11:659963. doi: 10.3389/fonc.2021.659963. eCollection 2021. Front Oncol. 2021. PMID: 33987095 Free PMC article. Review.
-
CircLIFR synergizes with MSH2 to attenuate chemoresistance via MutSα/ATM-p73 axis in bladder cancer.Mol Cancer. 2021 Apr 19;20(1):70. doi: 10.1186/s12943-021-01360-4. Mol Cancer. 2021. PMID: 33874956 Free PMC article.
-
Epigenetic control of the vicious cycle.J Bone Oncol. 2024 Jan 12;44:100524. doi: 10.1016/j.jbo.2024.100524. eCollection 2024 Feb. J Bone Oncol. 2024. PMID: 38304486 Free PMC article.
-
Skeletal impact of 17β-estradiol in T cell-deficient mice: age-dependent bone effects and osteosarcoma formation.Clin Exp Metastasis. 2020 Apr;37(2):269-281. doi: 10.1007/s10585-019-10012-3. Epub 2019 Dec 20. Clin Exp Metastasis. 2020. PMID: 31863240 Free PMC article.
-
Bone Tropism in Cancer Metastases.Cold Spring Harb Perspect Med. 2020 Oct 1;10(10):a036848. doi: 10.1101/cshperspect.a036848. Cold Spring Harb Perspect Med. 2020. PMID: 31615871 Free PMC article. Review.
References
-
- Croucher PI, McDonald MM, Martin TJ. Bone metastasis: the importance of the neighbourhood. Nature reviews Cancer. 2016;16:373–386. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

