Axial tubule junctions control rapid calcium signaling in atria
- PMID: 27643434
- PMCID: PMC5096811
- DOI: 10.1172/JCI88241
Axial tubule junctions control rapid calcium signaling in atria
Abstract
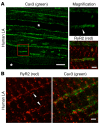
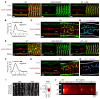
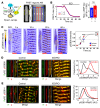
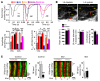
The canonical atrial myocyte (AM) is characterized by sparse transverse tubule (TT) invaginations and slow intracellular Ca2+ propagation but exhibits rapid contractile activation that is susceptible to loss of function during hypertrophic remodeling. Here, we have identified a membrane structure and Ca2+-signaling complex that may enhance the speed of atrial contraction independently of phospholamban regulation. This axial couplon was observed in human and mouse atria and is composed of voluminous axial tubules (ATs) with extensive junctions to the sarcoplasmic reticulum (SR) that include ryanodine receptor 2 (RyR2) clusters. In mouse AM, AT structures triggered Ca2+ release from the SR approximately 2 times faster at the AM center than at the surface. Rapid Ca2+ release correlated with colocalization of highly phosphorylated RyR2 clusters at AT-SR junctions and earlier, more rapid shortening of central sarcomeres. In contrast, mice expressing phosphorylation-incompetent RyR2 displayed depressed AM sarcomere shortening and reduced in vivo atrial contractile function. Moreover, left atrial hypertrophy led to AT proliferation, with a marked increase in the highly phosphorylated RyR2-pS2808 cluster fraction, thereby maintaining cytosolic Ca2+ signaling despite decreases in RyR2 cluster density and RyR2 protein expression. AT couplon "super-hubs" thus underlie faster excitation-contraction coupling in health as well as hypertrophic compensatory adaptation and represent a structural and metabolic mechanism that may contribute to contractile dysfunction and arrhythmias.
Figures




Comment in
-
Atrial-specific pathways for control of intracellular signaling and myocyte function.J Clin Invest. 2016 Oct 3;126(10):3731-3734. doi: 10.1172/JCI90348. Epub 2016 Sep 19. J Clin Invest. 2016. PMID: 27643440 Free PMC article.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials
Miscellaneous

