Immunogenicity and Lupus-Like Autoantibody Production Can Be Linked to Each Other along With Type I Interferon Production in Patients with Rheumatoid Arthritis Treated With Infliximab: A Retrospective Study of a Single Center Cohort
- PMID: 27643491
- PMCID: PMC5028026
- DOI: 10.1371/journal.pone.0162896
Immunogenicity and Lupus-Like Autoantibody Production Can Be Linked to Each Other along With Type I Interferon Production in Patients with Rheumatoid Arthritis Treated With Infliximab: A Retrospective Study of a Single Center Cohort
Abstract
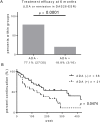
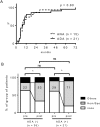
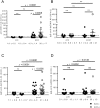
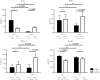
Besides anti-drug antibodies, anti-nuclear antibodies and anti-DNA antibodies are often induced in patients with rheumatoid arthritis treated with tumor necrosis factor inhibitors. We examined the association between immunogenicity, autoantibody production, and serum cytokine profiles in patients with rheumatoid arthritis treated with infliximab. Japanese patients with rheumatoid arthritis (n = 57) were retrospectively examined. Serum trough levels of infliximab, anti-drug antibody, anti-nuclear antibody, and anti-DNA (Farr), anti-single-stranded DNA and anti-double-stranded DNA antibodies were measured. Interleukin-6, interferon-γ, interferon-α, and B-cell activating factor levels were also measured in the same sera. Then, we validated the association between anti-drug antibody and these serum markers along with clinical response to infliximab. Anti-drug antibodies developed in twenty-one patients (36.8%), whose serum trough levels of infliximab were significantly lower than those in anti-drug antibody-negative patients (0.09 ± 0.03 vs. 2.48 ± 0.326 μg/mL, p < 0.0001). There were no significant differences in clinical backgrounds between the two groups. The anti-drug antibody-positive patients were more likely to develop anti-nuclear antibody titers of ≥ ×160 compared to the negative patients (14 to 57% vs. 17 to 33%). In addition, anti-DNA antibodies (Farr) (from 1.5 ± 0.4 to 35 ± 17 IU/mL, p = 0.0001), especially IgM-anti-double stranded DNA antibody (from 5.1 ± 0.7 to 41 ± 8.9 IU/mL, p < 0.0001), and IgG-anti-single stranded DNA antibody (from 13 ± 1.1 to 35 ± 13, p = 0.0145) were significantly increased in anti-drug antibody-positive but not in negative patients. Moreover, the anti-drug antibody-positive, but not the negative patients, showed significant increased levels of interferon-α (from 248.7 ± 102.3 to 466.8 ± 135.1 pg/mL, p = 0.0353) and B-cell activating factor (from 1073 ± 75.1 to 1387 ± 136.5 pg/mL, p = 0.0208) following infliximab treatment. The development of anti-drug antibody against infliximab and lupus-like autoantibody production in patients with rheumatoid arthritis treated with infliximab can be linked each other along with increased lupus-associated cytokine levels including type I interferons.
Conflict of interest statement
I have read the journal's policy and the authors of this manuscript have the following competing interests: T.F. has received grant and research support from Chugai Pharmaceutical Co., Ltd., Pfizer Japan Inc., and Ono Pharmaceutical Co., Ltd. The sponsors were not involved in the study design; in the collection, analysis, interpretation of data; in the writing of this manuscript; or in the decision to submit the article for publication. The authors, their immediate families, and any research foundations with which they are affiliated have not received any financial payments or other benefits from any commercial entity related to the subject of this article. This does not alter the authors' adherence to PLOS ONE policies on sharing data and materials.
Figures
Similar articles
-
Assessment of antibodies to double-stranded DNA induced in rheumatoid arthritis patients following treatment with infliximab, a monoclonal antibody to tumor necrosis factor alpha: findings in open-label and randomized placebo-controlled trials.Arthritis Rheum. 2000 Nov;43(11):2383-90. doi: 10.1002/1529-0131(200011)43:11<2383::AID-ANR2>3.0.CO;2-D. Arthritis Rheum. 2000. PMID: 11083258
-
Autoantibody formation in patients with rheumatoid arthritis treated with anti-TNF alpha.Ann Rheum Dis. 2005 Mar;64(3):403-7. doi: 10.1136/ard.2004.024182. Epub 2004 Aug 5. Ann Rheum Dis. 2005. PMID: 15297281 Free PMC article.
-
Induction of autoantibodies in refractory rheumatoid arthritis treated by infliximab.Clin Exp Rheumatol. 2004 Nov-Dec;22(6):756-8. Clin Exp Rheumatol. 2004. PMID: 15638051 Clinical Trial.
-
TNF alpha antagonist-induced lupus-like syndrome: report and review of the literature with implications for treatment with alternative TNF alpha antagonists.Int J Dermatol. 2011 May;50(5):619-25. doi: 10.1111/j.1365-4632.2011.04871.x. Int J Dermatol. 2011. PMID: 21506984 Review.
-
[The predictive value of autoantibodies in disseminating lupus erythematosus and rheumatoid arthritis].Ned Tijdschr Geneeskd. 2005 Mar 26;149(13):688-93. Ned Tijdschr Geneeskd. 2005. PMID: 15819133 Review. Dutch.
Cited by
-
Immunoassay methods used in clinical studies for the detection of anti-drug antibodies to adalimumab and infliximab.Clin Exp Immunol. 2018 Jun;192(3):348-365. doi: 10.1111/cei.13112. Epub 2018 Mar 30. Clin Exp Immunol. 2018. PMID: 29431871 Free PMC article. Review.
-
Recent Advances in Research Regarding Autoantibodies in Connective Tissue Diseases and Related Disorders.Intern Med. 2019 Jan 1;58(1):5-14. doi: 10.2169/internalmedicine.1423-18. Epub 2018 Aug 24. Intern Med. 2019. PMID: 30146596 Free PMC article. Review.
-
CD4+ T-Cell Dysfunction in Severe COVID-19 Disease Is Tumor Necrosis Factor-α/Tumor Necrosis Factor Receptor 1-Dependent.Am J Respir Crit Care Med. 2022 Jun 15;205(12):1403-1418. doi: 10.1164/rccm.202111-2493OC. Am J Respir Crit Care Med. 2022. PMID: 35348444 Free PMC article.
-
Drug-induced Bullous Pemphigoid and Lupus Erythematosus Occurring under Anti-TNF-α and IL-6 Therapy in a Patient with Rheumatoid Arthritis.Intern Med. 2020 Oct 15;59(20):2611-2618. doi: 10.2169/internalmedicine.4646-20. Epub 2020 Jul 7. Intern Med. 2020. PMID: 32641647 Free PMC article.
-
Presence of anti-nuclear antibodies is a risk factor for the appearance of anti-drug antibodies during infliximab or adalimumab therapy in patients with rheumatoid arthritis.PLoS One. 2020 Dec 14;15(12):e0243729. doi: 10.1371/journal.pone.0243729. eCollection 2020. PLoS One. 2020. PMID: 33315881 Free PMC article.
References
-
- Weinblatt ME, Keystone EC, Furst DE, Moreland LW, Weisman MH, Birbara CA, et al. Adalimumab, a fully human anti-tumor necrosis factor alpha monoclonal antibody, for the treatment of rheumatoid arthritis in patients taking concomitant methotrexate: the ARMADA trial. Arthritis Rheum. 2003;48:35–45. 10.1002/art.10697 . - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

