In Vitro Structural and Functional Characterization of the Small Heat Shock Proteins (sHSP) of the Cyanophage S-ShM2 and Its Host, Synechococcus sp. WH7803
- PMID: 27643500
- PMCID: PMC5028025
- DOI: 10.1371/journal.pone.0162233
In Vitro Structural and Functional Characterization of the Small Heat Shock Proteins (sHSP) of the Cyanophage S-ShM2 and Its Host, Synechococcus sp. WH7803
Abstract
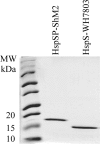
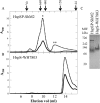
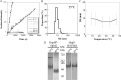
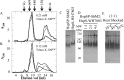
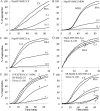
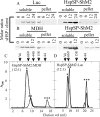
We previously reported the in silico characterization of Synechococcus sp. phage 18 kDa small heat shock protein (HspSP-ShM2). This small heat shock protein (sHSP) contains a highly conserved core alpha crystalline domain of 92 amino acids and relatively short N- and C-terminal arms, the later containing the classical C-terminal anchoring module motif (L-X-I/L/V). Here we establish the oligomeric profile of HspSP-ShM2 and its structural dynamics under in vitro experimental conditions using size exclusion chromatography (SEC/FPLC), gradient native gels electrophoresis and dynamic light scattering (DLS). Under native conditions, HspSP-ShM2 displays the ability to form large oligomers and shows a polydisperse profile. At higher temperatures, it shows extensive structural dynamics and undergoes conformational changes through an increased of subunit rearrangement and formation of sub-oligomeric species. We also demonstrate its capacity to prevent the aggregation of citrate synthase, malate dehydrogenase and luciferase under heat shock conditions through the formation of stable and soluble hetero-oligomeric complexes (sHSP:substrate). In contrast, the host cyanobacteria Synechococcus sp. WH7803 15 kDa sHSP (HspS-WH7803) aggregates when in the same conditions as HspSP-ShM2. However, its solubility can be maintained in the presence of non-ionic detergent Triton™X-100 and forms an oligomeric structure estimated to be between dimer and tetramer but exhibits no apparent inducible structural dynamics neither chaperon-like activity in all the assays and molar ratios tested. SEC/FPLC and thermal aggregation prevention assays results indicate no formation of hetero-oligomeric complex or functional interactions between both sHSPs. Taken together these in vitro results portray the phage HspSP-ShM2 as a classical sHSP and suggest that it may be functional at the in vivo level while behaving differently than its host amphitropic sHSP.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
References
-
- Van Montfort R, Slingsby C, Vierling E. Structure and function of the small heat shock protein/alpha-crystallin family of molecular chaperones. Adv Protein Chem. 2001;59:105–56. Epub 2002/03/01. . - PubMed
-
- de Jong WW, Caspers GJ, Leunissen JA. Genealogy of the alpha-crystallin—small heat-shock protein superfamily. Int J Biol Macromol. 1998;22(3–4):151–62. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

