Accumulation of Ubiquitin and Sequestosome-1 Implicate Protein Damage in Diacetyl-Induced Cytotoxicity
- PMID: 27643531
- PMCID: PMC5222965
- DOI: 10.1016/j.ajpath.2016.07.018
Accumulation of Ubiquitin and Sequestosome-1 Implicate Protein Damage in Diacetyl-Induced Cytotoxicity
Abstract
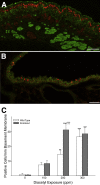
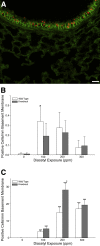
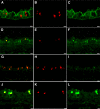
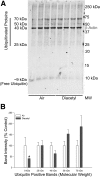
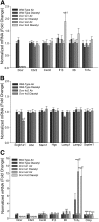
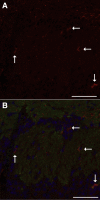
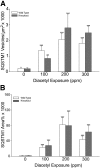
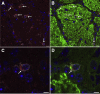
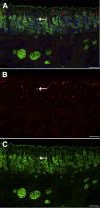
Inhaled diacetyl vapors are associated with flavorings-related lung disease, a potentially fatal airway disease. The reactive α-dicarbonyl group in diacetyl causes protein damage in vitro. Dicarbonyl/l-xylulose reductase (DCXR) metabolizes diacetyl into acetoin, which lacks this α-dicarbonyl group. To investigate the hypothesis that flavorings-related lung disease is caused by in vivo protein damage, we correlated diacetyl-induced airway damage in mice with immunofluorescence for markers of protein turnover and autophagy. Western immunoblots identified shifts in ubiquitin pools. Diacetyl inhalation caused dose-dependent increases in bronchial epithelial cells with puncta of both total ubiquitin and K63-ubiquitin, central mediators of protein turnover. This response was greater in Dcxr-knockout mice than in wild-type controls inhaling 200 ppm diacetyl, further implicating the α-dicarbonyl group in protein damage. Western immunoblots demonstrated decreased free ubiquitin in airway-enriched fractions. Transmission electron microscopy and colocalization of ubiquitin-positive puncta with lysosomal-associated membrane proteins 1 and 2 and with the multifunctional scaffolding protein sequestosome-1 (SQSTM1/p62) confirmed autophagy. Surprisingly, immunoreactive SQSTM1 also accumulated in the olfactory bulb of the brain. Olfactory bulb SQSTM1 often congregated in activated microglial cells that also contained olfactory marker protein, indicating neuronophagia within the olfactory bulb. This suggests the possibility that SQSTM1 or damaged proteins may be transported from the nose to the brain. Together, these findings strongly implicate widespread protein damage in the etiology of flavorings-related lung disease.
Copyright © 2016 American Society for Investigative Pathology. Published by Elsevier Inc. All rights reserved.
Figures

References
-
- Kreiss K., Gomaa A., Kullman G., Fedan K., Simoes E.J., Enright P.L. Clinical bronchiolitis obliterans in workers at a microwave-popcorn plant. N Engl J Med. 2002;347:330–338. - PubMed
-
- Hubbs A.F., Battelli L.A., Goldsmith W.T., Porter D.W., Frazer D., Friend S., Schwegler-Berry D., Mercer R.R., Reynolds J.S., Grote A., Castranova V., Kullman G., Fedan J.S., Dowdy J., Jones W.G. Necrosis of nasal and airway epithelium in rats inhaling vapors of artificial butter flavoring. Toxicol Appl Pharmacol. 2002;185:128–135. - PubMed
-
- Hubbs A.F., Goldsmith W.T., Kashon M.L., Frazer D., Mercer R.R., Battelli L.A., Kullman G.J., Schwegler-Berry D., Friend S., Castranova V. Respiratory toxicologic pathology of inhaled diacetyl in Sprague-Dawley rats. Toxicol Pathol. 2008;36:330–344. - PubMed
-
- Akpinar-Elci M., Travis W.D., Lynch D.A., Kreiss K. Bronchiolitis obliterans syndrome in popcorn production plant workers. Eur Respir J. 2004;24:298–302. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Miscellaneous

