Effects of vilazodone on sexual functioning in healthy adults: results from a randomized, double-blind, placebo-controlled, and active-controlled study
- PMID: 27643885
- PMCID: PMC5131696
- DOI: 10.1097/YIC.0000000000000145
Effects of vilazodone on sexual functioning in healthy adults: results from a randomized, double-blind, placebo-controlled, and active-controlled study
Abstract
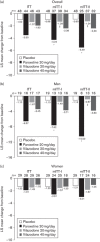
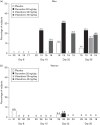
The aim of this study is to evaluate the effects of vilazodone on sexual functioning in healthy, sexually active adults and assess the impact of medication nonadherence in this type of trial. Participants were randomized to vilazodone (20 or 40 mg/day), paroxetine (20 mg/day), or placebo for 5 weeks of double-blind treatment. The primary endpoint was change from baseline to day 35 in Change in Sexual Functioning Questionnaire (CSFQ) total score in the intent-to-treat (ITT) population. Post-hoc analyses were carried out in modified intent-to-treat (mITT) populations that excluded participants in the active-treatment groups with undetectable plasma drug concentrations at all visits (mITT-I) or at least one visit (mITT-II). In the ITT population (N=199), there were no statistically significant differences between any treatment groups for CSFQ total score change: placebo, -1.0; vilazodone 20 mg/day, -1.4; vilazodone 40 mg/day, -1.9; and paroxetine, -3.5. In mITT-I (N=197) and mITT-II (N=159), CSFQ total score change was not significantly different between vilazodone (either dose) versus placebo; the CSFQ total score decreased significantly (P<0.05) with paroxetine versus both placebo and vilazodone 20 mg/day, but not versus vilazodone 40 mg/day. Vilazodone exerted no significant effect on sexual functioning in healthy adults. Medication nonadherence can alter study results and may be an important consideration in trials with volunteer participants.
Figures

References
-
- APA (2013). Diagnostic and Statistical Manual of Mental Disorders, Fifth Edition. Arlington, VA: American Psychiatric Association.
-
- Beutel ME, Stöbel-Richter Y, Brähler E. (2008). Sexual desire and sexual activity of men and women across their lifespans: results from a representative German community survey. BJU Int 101:76–82. - PubMed
-
- Bijlsma EY, Chan JS, Olivier B, Veening JG, Millan MJ, Waldinger MD, Oosting RS. (2014). Sexual side effects of serotonergic antidepressants: mediated by inhibition of serotonin on central dopamine release? Pharmacol Biochem Behav 121:88–101. - PubMed
-
- Clayton AH, McGarvey EL, Clavet GJ. (1997a). The Changes in Sexual Functioning Questionnaire (CSFQ): development, reliability, and validity. Psychopharmacol Bull 33:731–745. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

