Acute Acrolein Exposure Induces Impairment of Vocal Fold Epithelial Barrier Function
- PMID: 27643990
- PMCID: PMC5028054
- DOI: 10.1371/journal.pone.0163237
Acute Acrolein Exposure Induces Impairment of Vocal Fold Epithelial Barrier Function
Abstract
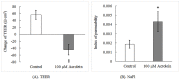
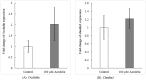
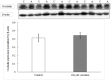
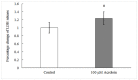
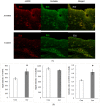
Acrolein is a ubiquitous pollutant abundant in cigarette smoke, mobile exhaust, and industrial waste. There is limited literature on the effects of acrolein on vocal fold tissue, although there are clinical reports of voice changes after pollutant exposures. Vocal folds are responsible for voice production. The overall objective of this study was to investigate the effects of acrolein exposure on viable, excised vocal fold epithelial tissue and to characterize the mechanism underlying acrolein toxicity. Vocal fold epithelia were studied because they form the outermost layer of the vocal folds and are a primary recipient of inhaled pollutants. Porcine vocal fold epithelia were exposed to 0, 50, 100, 500, 900 or 1300 μM of acrolein for 3 hours; the metabolic activity, epithelial resistance, epithelial permeability, tight junction protein (occludin and claudin 3) expression, cell membrane integrity and lipid peroxidation were investigated. The data demonstrated that acrolein exposure at 500 μM significantly reduced vocal fold epithelial metabolic activity by 27.2% (p≤0.001). Incubation with 100 μM acrolein caused a marked increase in epithelial permeability by 130.5% (p<0.05) and a reduction in transepithelial electrical resistance (TEER) by 180.0% (p<0.001). While the expression of tight junctional protein did not change in acrolein-treated samples, the cell membrane integrity was significantly damaged with a 45.6% increase of lipid peroxidation as compared to controls (p<0.05). Taken together, these data provide evidence that acute acrolein exposure impairs vocal fold epithelial barrier integrity. Lipid peroxidation-induced cell membrane damage may play an important role in reducing the barrier function of the epithelium.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures

Similar articles
-
Vocal fold ion transport and mucin expression following acrolein exposure.J Membr Biol. 2014 May;247(5):441-50. doi: 10.1007/s00232-014-9651-2. Epub 2014 Mar 20. J Membr Biol. 2014. PMID: 24648011 Free PMC article.
-
Hypertonic challenge to porcine vocal folds: effects on epithelial barrier function.Otolaryngol Head Neck Surg. 2010 Jan;142(1):79-84. doi: 10.1016/j.otohns.2009.09.011. Epub 2009 Nov 22. Otolaryngol Head Neck Surg. 2010. PMID: 20096227 Free PMC article.
-
Subacute acrolein exposure to rat larynx in vivo.Laryngoscope. 2019 Sep;129(9):E313-E317. doi: 10.1002/lary.27687. Epub 2018 Dec 24. Laryngoscope. 2019. PMID: 30582162 Free PMC article.
-
Utility of cell viability assays for use with ex vivo vocal fold epithelial tissue.Laryngoscope. 2015 May;125(5):E180-5. doi: 10.1002/lary.25100. Epub 2014 Dec 15. Laryngoscope. 2015. PMID: 25511412 Free PMC article.
-
Vocal fold epithelial barrier in health and injury: a research review.J Speech Lang Hear Res. 2014 Oct;57(5):1679-91. doi: 10.1044/2014_JSLHR-S-13-0283. J Speech Lang Hear Res. 2014. PMID: 24686981 Free PMC article. Review.
Cited by
-
Acute Nanoparticle Exposure to Vocal Folds: A Laboratory Study.J Voice. 2017 Nov;31(6):662-668. doi: 10.1016/j.jvoice.2017.03.014. Epub 2017 Apr 21. J Voice. 2017. PMID: 28438490 Free PMC article.
-
Cigarette Smoke Exposure to Pig Larynx in an Inhalation Chamber.J Voice. 2019 Nov;33(6):846-850. doi: 10.1016/j.jvoice.2018.05.005. Epub 2018 Jul 5. J Voice. 2019. PMID: 29983221 Free PMC article.
-
Protective Effect of Purinergic P2X7 Receptor Inhibition on Acrolein-Induced Urothelial Cell Damage.Front Physiol. 2022 Apr 12;13:885545. doi: 10.3389/fphys.2022.885545. eCollection 2022. Front Physiol. 2022. PMID: 35492615 Free PMC article.
-
Acrolein Promotes Aging and Oxidative Stress via the Stress Response Factor DAF-16/FOXO in Caenorhabditis elegans.Foods. 2022 May 28;11(11):1590. doi: 10.3390/foods11111590. Foods. 2022. PMID: 35681340 Free PMC article.
-
Scalable and High-Throughput In Vitro Vibratory Platform for Vocal Fold Tissue Engineering Applications.Bioengineering (Basel). 2023 May 17;10(5):602. doi: 10.3390/bioengineering10050602. Bioengineering (Basel). 2023. PMID: 37237672 Free PMC article.
References
-
- Sivasankar M, Fisher KV. Vocal fold epithelial response to luminal osmotic perturbation. J Speech Lang Hear Res. 2007; 50: 886–898. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

