Rapid hepatic clearance of full length CCN-2/CTGF: a putative role for LRP1-mediated endocytosis
- PMID: 27644406
- PMCID: PMC5143326
- DOI: 10.1007/s12079-016-0354-6
Rapid hepatic clearance of full length CCN-2/CTGF: a putative role for LRP1-mediated endocytosis
Abstract
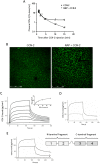
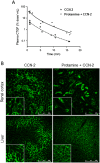
CCN-2 (connective tissue growth factor; CTGF) is a key factor in fibrosis. Plasma CCN-2 has biomarker potential in numerous fibrotic disorders, but it is unknown which pathophysiological factors determine plasma CCN-2 levels. The proteolytic amino-terminal fragment of CCN-2 is primarily eliminated by the kidney. Here, we investigated elimination and distribution profiles of full length CCN-2 by intravenous administration of recombinant CCN-2 to rodents. After bolus injection in mice, we observed a large initial distribution volume (454 mL/kg) and a fast initial clearance (120 mL/kg/min). Immunosorbent assay and immunostaining showed that CCN-2 distributed mainly to the liver and was taken up by hepatocytes. Steady state clearance in rats, determined by continuous infusion of CCN-2, was fast (45 mL/kg/min). Renal CCN-2 clearance, determined by arterial and renal vein sampling, accounted for only 12 % of total clearance. Co-infusion of CCN-2 with receptor-associated protein (RAP), an antagonist of LDL-receptor family proteins, showed that RAP prolonged CCN-2 half-life and completely prevented CCN-2 internalization by hepatocytes. This suggests that hepatic uptake of CCN-2 is mediated by a RAP-sensitive mechanism most likely involving LRP1, a member of the LDL-receptor family involved in hepatic clearance of various plasma proteins. Surface plasmon resonance binding studies confirmed that CCN-2 is an LRP1 ligand. Co-infusion of CCN-2 with an excess of the heparan sulphate-binding protamine lowered the large initial distribution volume of CCN-2 by 88 % and reduced interstitial staining of CCN-2, suggesting binding of CCN-2 to heparan sulphate proteoglycans (HSPGs). Protamine did not affect clearance rate, indicating that RAP-sensitive clearance of CCN-2 is HSPG independent. In conclusion, unlike its amino-terminal fragment which is cleared by the kidney, full length CCN-2 is primarily eliminated by the liver via a fast RAP-sensitive, probably LRP1-dependent pathway.
Keywords: Biomarker; CCN-2; CTGF; Hepatic clearance; LRP1.
Conflict of interest statement
Compliance with ethical standards Disclosures This study was supported by a grant from FibroGen, Inc., a company interested in commercializing anti-CCN-2 therapies. RG has been employed by and has received research support from FibroGen.
Figures

Similar articles
-
The adaptor protein PID1 regulates receptor-dependent endocytosis of postprandial triglyceride-rich lipoproteins.Mol Metab. 2018 Oct;16:88-99. doi: 10.1016/j.molmet.2018.07.010. Epub 2018 Jul 30. Mol Metab. 2018. PMID: 30100244 Free PMC article.
-
Renal proximal tubular dysfunction is a major determinant of urinary connective tissue growth factor excretion.Am J Physiol Renal Physiol. 2010 Jun;298(6):F1457-64. doi: 10.1152/ajprenal.00694.2009. Epub 2010 Mar 17. Am J Physiol Renal Physiol. 2010. PMID: 20237235
-
The hypolipidemic effect of cilostazol can be mediated by regulation of hepatic low-density lipoprotein receptor-related protein 1 (LRP1) expression.Metabolism. 2014 Jan;63(1):112-9. doi: 10.1016/j.metabol.2013.09.006. Epub 2013 Oct 17. Metabolism. 2014. PMID: 24139096
-
Cell surface receptors for CCN proteins.J Cell Commun Signal. 2016 Jun;10(2):121-7. doi: 10.1007/s12079-016-0324-z. Epub 2016 Apr 20. J Cell Commun Signal. 2016. PMID: 27098435 Free PMC article. Review.
-
Matricellular proteins of the Cyr61/CTGF/NOV (CCN) family and the nervous system.Front Cell Neurosci. 2015 Jun 24;9:237. doi: 10.3389/fncel.2015.00237. eCollection 2015. Front Cell Neurosci. 2015. PMID: 26157362 Free PMC article. Review.
Cited by
-
CCN Family Proteins in Cancer: Insight Into Their Structures and Coordination Role in Tumor Microenvironment.Front Genet. 2021 Mar 23;12:649387. doi: 10.3389/fgene.2021.649387. eCollection 2021. Front Genet. 2021. PMID: 33833779 Free PMC article. Review.
-
Structural insights into regulation of CCN protein activities and functions.J Cell Commun Signal. 2023 Jun;17(2):371-390. doi: 10.1007/s12079-023-00768-5. Epub 2023 May 28. J Cell Commun Signal. 2023. PMID: 37245184 Free PMC article. Review.
-
The von Willebrand factor A-1 domain binding aptamer BT200 elevates plasma levels of von Willebrand factor and factor VIII: a first-in-human trial.Haematologica. 2022 Sep 1;107(9):2121-2132. doi: 10.3324/haematol.2021.279948. Haematologica. 2022. PMID: 34818873 Free PMC article. Clinical Trial.
-
Thrombospondin 1 and Its Diverse Roles as a Regulator of Extracellular Matrix in Fibrotic Disease.J Histochem Cytochem. 2019 Sep;67(9):683-699. doi: 10.1369/0022155419851103. Epub 2019 May 22. J Histochem Cytochem. 2019. PMID: 31116066 Free PMC article. Review.
-
Rescue of murine hind limb ischemia via angiogenesis and lymphangiogenesis promoted by cellular communication network factor 2.Sci Rep. 2023 Nov 16;13(1):20029. doi: 10.1038/s41598-023-47485-y. Sci Rep. 2023. PMID: 37973852 Free PMC article.
References
-
- Bauer S, Eisinger K, Wiest R, Karrasch T, Scherer MN, Farkas S, Aslanidis C, Buechler C. Connective tissue growth factor level is increased in patients with liver cirrhosis but is not associated with complications or extent of liver injury. Regul Pept. 2012;179:10–14. doi: 10.1016/j.regpep.2012.08.007. - DOI - PubMed
-
- Cicha I, Garlichs CD, Daniel WG, Goppelt-Struebe M. Activated human platelets release connective tissue growth factor. Thromb Haemost. 2004;91:755–760. - PubMed
-
- Colak Y, Senates E, Coskunpinar E, Oltulu YM, Zemheri E, Ozturk O, Doganay L, Mesci B, Yilmaz Y, Enc FY, Kiziltas S, Ulasoglu C, Tuncer I. Concentrations of connective tissue growth factor in patients with nonalcoholic fatty liver disease: association with liver fibrosis. Dis Markers. 2012;33:77–83. doi: 10.1155/2012/283726. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

