Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile
- PMID: 27645238
- PMCID: PMC5118987
- DOI: 10.1128/AAC.01474-16
Antiviral Activity of Bictegravir (GS-9883), a Novel Potent HIV-1 Integrase Strand Transfer Inhibitor with an Improved Resistance Profile
Abstract
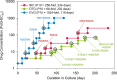
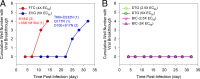
Bictegravir (BIC; GS-9883), a novel, potent, once-daily, unboosted inhibitor of HIV-1 integrase (IN), specifically targets IN strand transfer activity (50% inhibitory concentration [IC50] of 7.5 ± 0.3 nM) and HIV-1 integration in cells. BIC exhibits potent and selective in vitro antiretroviral activity in both T-cell lines and primary human T lymphocytes, with 50% effective concentrations ranging from 1.5 to 2.4 nM and selectivity indices up to 8,700 relative to cytotoxicity. BIC exhibits synergistic in vitro antiviral effects in pairwise combinations with tenofovir alafenamide, emtricitabine, or darunavir and maintains potent antiviral activity against HIV-1 variants resistant to other classes of antiretrovirals. BIC displayed an in vitro resistance profile that was markedly improved compared to the integrase strand transfer inhibitors (INSTIs) raltegravir (RAL) and elvitegravir (EVG), and comparable to that of dolutegravir (DTG), against nine INSTI-resistant site-directed HIV-1 mutants. BIC displayed statistically improved antiviral activity relative to EVG, RAL, and DTG against a panel of 47 patient-derived HIV-1 isolates with high-level INSTI resistance; 13 of 47 tested isolates exhibited >2-fold lower resistance to BIC than DTG. In dose-escalation experiments conducted in vitro, BIC and DTG exhibited higher barriers to resistance than EVG, selecting for HIV-1 variants with reduced phenotypic susceptibility at days 71, 87, and 20, respectively. A recombinant virus with the BIC-selected M50I/R263K dual mutations in IN exhibited only 2.8-fold reduced susceptibility to BIC compared to wild-type virus. All BIC-selected variants exhibited low to intermediate levels of cross-resistance to RAL, DTG, and EVG (<8-fold) but remained susceptible to other classes of antiretrovirals. A high barrier to in vitro resistance emergence for both BIC and DTG was also observed in viral breakthrough studies in the presence of constant clinically relevant drug concentrations. The overall virologic profile of BIC supports its ongoing clinical investigation in combination with other antiretroviral agents for both treatment-naive and -experienced HIV-infected patients.
Copyright © 2016 Tsiang et al.
Figures

References
-
- Cooper DA, Steigbigel RT, Gatell J, Rockstroh J, Katlama C, Yeni P, Lazzarin A, Clotet B, Kumar P, Eron JE, Schechter M, Markowitz M, Loutfy MR, Lennox JL, Zhao J, Chen J, Ryan DM, Rhodes RR, Killar JA, Gilde LR, Strohmaier KM, Meibohm AR, Miller MD, Hazuda DJ, Nessly ML, DiNubile MJ, Isaacs RD, Teppler H, Nguyen B. 2008. Subgroup and resistance analyses of raltegravir for resistant HIV-1 infection. N Engl J Med 359:355–365. doi: 10.1056/NEJMoa0708978. - DOI - PubMed
-
- Markowitz M, Nguyen BY, Gotuzzo E, Mendo F, Ratanasuwan W, Kovacs C, Prada G, Morales-Ramirez JO, Crumpacker CS, Isaacs RD, Campbell H, Strohmaier KM, Wan H, Danovich RM, Teppler H. 2009. Sustained antiretroviral effect of raltegravir after 96 weeks of combination therapy in treatment-naive patients with HIV-1 infection. J Acquir Immune Defic Syndr 52:350–356. doi: 10.1097/QAI.0b013e3181b064b0. - DOI - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

