Recruitment and representativeness of blood donors in the INTERVAL randomised trial assessing varying inter-donation intervals
- PMID: 27645285
- PMCID: PMC5029021
- DOI: 10.1186/s13063-016-1579-7
Recruitment and representativeness of blood donors in the INTERVAL randomised trial assessing varying inter-donation intervals
Abstract
Background: The interpretation of trial results can be helped by understanding how generalisable they are to the target population for which inferences are intended. INTERVAL, a large pragmatic randomised trial of blood donors in England, is assessing the effectiveness and safety of reducing inter-donation intervals. The trial recruited mainly from the blood service's static centres, which collect only about 10 % of whole-blood donations. Hence, the extent to which the trial's participants are representative of the general blood donor population is uncertain. We compare these groups in detail.
Methods: We present the CONSORT flowchart from participant invitation to randomisation in INTERVAL. We compare the characteristics of those eligible and consenting to participate in INTERVAL with the general donor population, using the national blood supply 'PULSE' database for the period of recruitment. We compare the characteristics of specific groups of trial participants recruited from different sources, as well as those who were randomised versus those not randomised.
Results: From a total of 540,459 invitations, 48,725 donors were eligible and consented to participate in INTERVAL. The proportion of such donors varied from 1-22 % depending on the source of recruitment. The characteristics of those consenting were similar to those of the general population of 1.3 million donors in terms of ethnicity, blood group distribution and recent deferral rates from blood donation due to low haemoglobin. However, INTERVAL participants included more men (50 % versus 44 %), were slightly older (mean age 43.1 versus 42.3 years), included fewer new donors (3 % versus 22 %) and had given more donations over the previous 2 years (mean 3.3 versus 2.2) than the general donor population. Of the consenting participants, 45,263 (93 %) donors were randomised. Compared to those not randomised, the randomised donors showed qualitatively similar differences to those described above.
Conclusions: There was broad similarity of participants in INTERVAL with the general blood donor population of England, notwithstanding some differences in age, sex and donation history. Any heterogeneity of the trial's results according to these characteristics will need to be studied to ensure its generalisability to the general donor population.
Trial registration: Current Controlled Trials ISRCTN24760606 . Registered on 25 January 2012.
Keywords: Blood donation; Blood donors; Generalisability; Randomised trial; Recruitment; Representativeness.
Figures


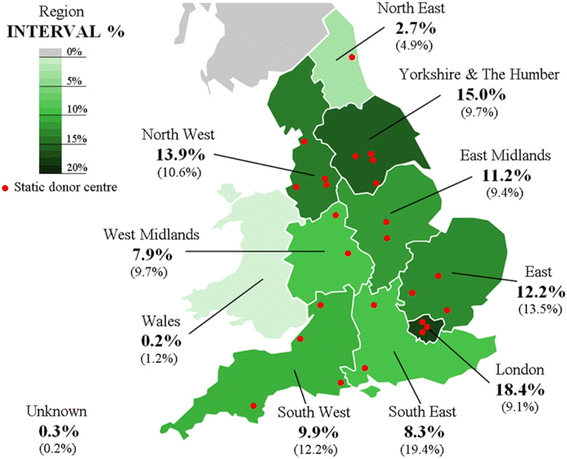
References
-
- Moore C, Sambrook J, Walker M, Tolkien Z, Kaptoge S, Allen D, Mehenny S, Mant J, Di Angelantonio E, Thompson SG, et al. The INTERVAL trial to determine whether intervals between blood donations can be safely and acceptably decreased to optimise blood supply: study protocol for a randomised controlled trial. Trials. 2014;15:363. doi: 10.1186/1745-6215-15-363. - DOI - PMC - PubMed
-
- NHS Blood and Transplant. Blood 2020. A strategy for the blood supply in England and North Wales. http://www.nhsbt.nhs.uk/download/blood-2020.pdf. Accessed 6 May 2016.
-
- Fendrich K, Hoffmann W. More than just aging societies: the demographic change has an impact on actual numbers of patients. J Public Health. 2007;15(5):345–51. doi: 10.1007/s10389-007-0142-0. - DOI
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

