Room-Temperature All-solid-state Rechargeable Sodium-ion Batteries with a Cl-doped Na3PS4 Superionic Conductor
- PMID: 27645565
- PMCID: PMC5028709
- DOI: 10.1038/srep33733
Room-Temperature All-solid-state Rechargeable Sodium-ion Batteries with a Cl-doped Na3PS4 Superionic Conductor
Abstract
All-solid-state sodium-ion batteries are promising candidates for large-scale energy storage applications. The key enabler for an all-solid-state architecture is a sodium solid electrolyte that exhibits high Na(+) conductivity at ambient temperatures, as well as excellent phase and electrochemical stability. In this work, we present a first-principles-guided discovery and synthesis of a novel Cl-doped tetragonal Na3PS4 (t-Na3-xPS4-xClx) solid electrolyte with a room-temperature Na(+) conductivity exceeding 1 mS cm(-1). We demonstrate that an all-solid-state TiS2/t-Na3-xPS4-xClx/Na cell utilizing this solid electrolyte can be cycled at room-temperature at a rate of C/10 with a capacity of about 80 mAh g(-1) over 10 cycles. We provide evidence from density functional theory calculations that this excellent electrochemical performance is not only due to the high Na(+) conductivity of the solid electrolyte, but also due to the effect that "salting" Na3PS4 has on the formation of an electronically insulating, ionically conducting solid electrolyte interphase.
Figures




 ) and NaCl (
) and NaCl ( ). The difference between the observed and calculated patterns is signified by the blue line. (d) SEM image of pristine t-Na3PS4 SPS sample, and (e) SEM image of SPS sample of doped t-Na3−xPS4−xClx (x = 6.25%). Scale bar is 10 μm.
). The difference between the observed and calculated patterns is signified by the blue line. (d) SEM image of pristine t-Na3PS4 SPS sample, and (e) SEM image of SPS sample of doped t-Na3−xPS4−xClx (x = 6.25%). Scale bar is 10 μm.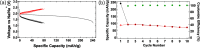
References
-
- Hueso K. B., Armand M. & Rojo T. High temperature sodium batteries: status, challenges and future trends. Energy Environ. Sci. 6, 734 (2013).
-
- Ellis B. L. & Nazar L. F. Sodium and sodium-ion energy storage batteries. Curr. Opin. Solid State Mater. Sci. 16, 168–177 (2012).
-
- Larcher D. & Tarascon J. Towards greener and more sustainable batteries for electrical energy storage. Nat. Chem. 7, 19–29 (2014). - PubMed
-
- Ong S. P. et al.. Voltage, stability and diffusion barrier differences between sodium-ion and lithium-ion intercalation materials. Energy Environ. Sci. 4, 3680 (2011).
-
- Tarascon J.-M. & Armand M. Issues and challenges facing rechargeable lithium batteries. Nature 414, 359–367 (2001). - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources

