The role of FDG PET/CT in patients treated with neoadjuvant chemotherapy for localized bone sarcomas
- PMID: 27645694
- PMCID: PMC5215266
- DOI: 10.1007/s00259-016-3509-z
The role of FDG PET/CT in patients treated with neoadjuvant chemotherapy for localized bone sarcomas
Abstract
Purpose: The histological response to neoadjuvant chemotherapy is an important prognostic factor in patients with osteosarcoma (OS) and Ewing sarcoma (EWS). The aim of this study was to assess baseline primary tumour FDG uptake on PET/CT, and serum values of alkaline phosphatase (ALP) and lactate dehydrogenase (LDH), to establish whether these factors are correlated with tumour necrosis and prognosis.
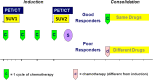
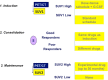
Methods: Patients treated between 2009 and 2014 for localized EWS and OS, who underwent FDG PET/CT as part of their staging work-up, were included. The relationships between primary tumour SUVmax at baseline (SUV1), SUVmax after induction chemotherapy (SUV2), metabolic response calculated as [(SUV1 - SUV2)/SUV1)] × 100, LDH and ALP and tumour response/survival were analysed. A good response (GR) was defined as tumour necrosis >90 % in patients with OS, and grade II-III Picci necrosis (persitence of microscopic foci only or no viable tumor) in patients with Ewing sarcoma.
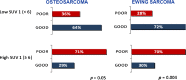
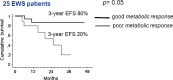
Results: The study included 77 patients, 45 with EWS and 32 with OS. A good histological response was achieved in 53 % of EWS patients, and 41 % of OS patients. The 3-year event-free survival (EFS) was 57 % in EWS patients and 48 % OS patients. The median SUV1 was 5.6 (range 0 - 17) in EWS patients and 7.9 (range 0 - 24) in OS patients (p = 0.006). In EWS patients the GR rate was 30 % in those with a high SUV1 (≥6) and 72 % in those with a lower SUV1 (p = 0.0004), and in OS patients the GR rate was 29 % in those with SUV1 ≥6 and 64 % in those with a lower SUV1 (p = 0.05). In the univariate analysis the 3-year EFS was significantly better in patients with a low ALP level (59 %) than in those with a high ALP level (22 %, p = 0.02) and in patients with a low LDH level (62 %) than in those with a high LDH level (37 %, p = 0.004). In EWS patients the 3-year EFS was 37 % in those with a high SUV1 and 75 % in those with a low SUV1 (p = 0.004), and in OS patients the 3-year EFS was 32 % in those with a high SUV1 and 66 % in those with a low SUV1 (p = 0.1). Histology, age and gender were not associated with survival. In the multivariate analysis, SUV1 was the only independent pretreatment prognostic factor to retain statistical significance (p = 0.017). SUV2 was assessed in 25 EWS patients: the median SUV2 was 1.9 (range 1 - 8). The GR rate was 20 % in patients with a high SUV2, and 67 % in those with a low SUV2 (p = 0.02). A good metabolic response (SUV reduction of ≥55 %) was associated with a 3-year EFS of 80 % and a poor metabolic response with a 3-year EFS of 20 % (p = 0.05). In the OS patients the median SUV2 was 2.7 (range 0 - 4.5). Neither SUV2 nor the metabolic response was associated with outcome in OS patients.
Conclusion: FDG PET/CT is a useful and noninvasive tool for identifying patients who are more likely to be resistant to chemotherapy. If this finding is confirmed in a larger series, SUV1, SUV2 and metabolic response could be proposed as factors for stratifying EWS patients to identify those with high-grade localized bone EWS who would benefit from risk-adapted induction chemotherapy.
Keywords: Ewing sarcoma; Neo-adjuvant chemotherapy; Osteosarcoma; PET-CT; Prognosis; SUV1.
Conflict of interest statement
Compliance with ethical standards Funding This work was supported by the Regione – Università Project, A. Liberati, Italian Ministry of Health – Project Alleanza contro il Cancro (ACC), an “Associazione Matteo Amitrano ONLUS” research grant, and an ESMO Congress Travel Grant. Conflict of interest None. Ethical approval All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the principles of the 1964 Declaration of Helsinki and its later amendments or comparable ethical standards.
Figures



References
-
- Ferrari S, Ruggieri P, Cefalo G, Tamburini A, Capanna R, Fagioli F, et al. Neoadjuvant chemotherapy with methotrexate, cisplatin, and doxorubicin with or without ifosfamide in nonmetastatic osteosarcoma of the extremity: an Italian Sarcoma Group trial ISG/OS-1. J Clin Oncol. 2012;17:2112–2118. doi: 10.1200/JCO.2011.38.4420. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

