Unbiased screen identifies aripiprazole as a modulator of abundance of the polyglutamine disease protein, ataxin-3
- PMID: 27645800
- PMCID: PMC5840879
- DOI: 10.1093/brain/aww228
Unbiased screen identifies aripiprazole as a modulator of abundance of the polyglutamine disease protein, ataxin-3
Abstract
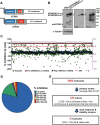
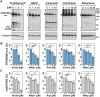
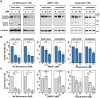
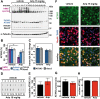
No disease-modifying treatment exists for the fatal neurodegenerative polyglutamine disease known both as Machado-Joseph disease and spinocerebellar ataxia type 3. As a potential route to therapy, we identified small molecules that reduce levels of the mutant disease protein, ATXN3. Screens of a small molecule collection, including 1250 Food and Drug Administration-approved drugs, in a novel cell-based assay, followed by secondary screens in brain slice cultures from transgenic mice expressing the human disease gene, identified the atypical antipsychotic aripiprazole as one of the hits. Aripiprazole increased longevity in a Drosophila model of Machado-Joseph disease and effectively reduced aggregated ATXN3 species in flies and in brains of transgenic mice treated for 10 days. The aripiprazole-mediated decrease in ATXN3 abundance may reflect a complex response culminating in the modulation of specific components of cellular protein homeostasis. Aripiprazole represents a potentially promising therapeutic drug for Machado-Joseph disease and possibly other neurological proteinopathies.
Keywords: Machado-Joseph disease; drug screen; neurodegeneration; spinocerebellar ataxia type 3; therapeutics.
© The Author (2016). Published by Oxford University Press on behalf of the Guarantors of Brain. All rights reserved. For Permissions, please email: journals.permissions@oup.com.
Figures




References
-
- Berger Z, Ravikumar B, Menzies FM, Oroz LG, Underwood BR, Pangalos MN, et al. Rapamycin alleviates toxicity of different aggregate-prone proteins. Hum Mol Genet 2006; 15: 433–42. - PubMed
-
- Boehmerle W, Muenzfeld H, Springer A, Huehnchen P, Endres M. Specific targeting of neurotoxic side effects and pharmacological profile of the novel cancer stem cell drug salinomycin in mice. J Mol Med 2014; 92: 889–900. - PubMed
-
- Cemal CK, Carroll CJ, Lawrence L, Lowrie MB, Ruddle P, Al-Mahdawi S, et al. YAC transgenic mice carrying pathological alleles of the MJD1 locus exhibit a mild and slowly progressive cerebellar deficit. Hum Mol Genet 2002; 11: 1075–94. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

