The transcription factor XBP1s restores hippocampal synaptic plasticity and memory by control of the Kalirin-7 pathway in Alzheimer model
- PMID: 27646263
- PMCID: PMC5658671
- DOI: 10.1038/mp.2016.152
The transcription factor XBP1s restores hippocampal synaptic plasticity and memory by control of the Kalirin-7 pathway in Alzheimer model
Abstract
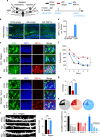
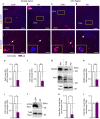
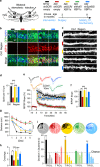
Neuronal network dysfunction and cognitive decline constitute the most prominent features of Alzheimer's disease (AD), although mechanisms causing such impairments are yet to be determined. Here we report that virus-mediated delivery of the active spliced transcription factor X-Box binding protein 1s (XBP1s) in the hippocampus rescued spine density, synaptic plasticity and memory function in a mouse model of AD. XBP1s transcriptionally activated Kalirin-7 (Kal7), a protein that controls synaptic plasticity. In addition, we found reduced levels of Kal7 in primary neurons exposed to Aβ oligomers, transgenic mouse models and human AD brains. Short hairpin RNA-mediated knockdown of Kal7 altered synaptic plasticity and memory formation in naive mice. Further, reduction of endogenous Kal7 compromised the beneficial effects of XBP1s in Alzheimer's model. Hence, our findings reveal that XBP1s is neuroprotective through a mechanism that engages Kal7 pathway with therapeutic implications in AD pathology.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Busquets-Garcia A, Gomis-Gonzalez M, Guegan T, Agustin-Pavon C, Pastor A, Mato S et al. Targeting the endocannabinoid system in the treatment of fragile X syndrome. Nat Med 2013; 19: 603–607. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

