Defective fluid shear stress mechanotransduction mediates hereditary hemorrhagic telangiectasia
- PMID: 27646277
- PMCID: PMC5037412
- DOI: 10.1083/jcb.201603106
Defective fluid shear stress mechanotransduction mediates hereditary hemorrhagic telangiectasia
Abstract
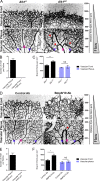
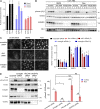
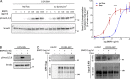
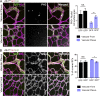
Morphogenesis of the vascular system is strongly modulated by mechanical forces from blood flow. Hereditary hemorrhagic telangiectasia (HHT) is an inherited autosomal-dominant disease in which arteriovenous malformations and telangiectasias accumulate with age. Most cases are linked to heterozygous mutations in Alk1 or Endoglin, receptors for bone morphogenetic proteins (BMPs) 9 and 10. Evidence suggests that a second hit results in clonal expansion of endothelial cells to form lesions with poor mural cell coverage that spontaneously rupture and bleed. We now report that fluid shear stress potentiates BMPs to activate Alk1 signaling, which correlates with enhanced association of Alk1 and endoglin. Alk1 is required for BMP9 and flow responses, whereas endoglin is only required for enhancement by flow. This pathway mediates both inhibition of endothelial proliferation and recruitment of mural cells; thus, its loss blocks flow-induced vascular stabilization. Identification of Alk1 signaling as a convergence point for flow and soluble ligands provides a molecular mechanism for development of HHT lesions.
© 2016 Baeyens et al.
Figures
References
-
- Arthur H.M., Ure J., Smith A.J., Renforth G., Wilson D.I., Torsney E., Charlton R., Parums D.V., Jowett T., Marchuk D.A., et al. 2000. Endoglin, an ancillary TGFbeta receptor, is required for extraembryonic angiogenesis and plays a key role in heart development. Dev. Biol. 217:42–53. 10.1006/dbio.1999.9534 - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

