Reduction of Isoagglutinin in Intravenous Immunoglobulin (IVIG) Using Blood Group A- and B-Specific Immunoaffinity Chromatography: Industry-Scale Assessment
- PMID: 27646589
- PMCID: PMC5054059
- DOI: 10.1007/s40259-016-0192-3
Reduction of Isoagglutinin in Intravenous Immunoglobulin (IVIG) Using Blood Group A- and B-Specific Immunoaffinity Chromatography: Industry-Scale Assessment
Abstract
Background: Hemolysis, a rare but potentially serious complication of intravenous immunoglobulin (IVIG) therapy, is associated with the presence of antibodies to blood groups A and B (isoagglutinins) in the IVIG product. An immunoaffinity chromatography (IAC) step in the production process could decrease isoagglutinin levels in IVIG.
Objectives: Our objectives were to compare isoagglutinin levels in a large number of IVIG (Privigen®) batches produced with or without IAC and to assess the feasibility of the production process with an IAC step on an industrial scale.
Methods: The IAC column comprised a blend of anti-A and anti-B resins formed by coupling synthetic blood group antigens (A/B-trisaccharides) to a base bead matrix, and was introduced towards the end of the industrial-scale IVIG manufacturing process. Isoagglutinin levels in IVIG were determined by anti-A and anti-B hemagglutinin direct and indirect methods according to the European Pharmacopoeia (Ph. Eur.) and an isoagglutinin flow cytometry assay. IVIG product quality was assessed with respect to the retention of immunoglobulin G (IgG) subclasses, specific antibodies, and removal of IgM using standardized procedures.
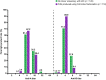
Results: The IAC step reduced isoagglutinins in IVIG by two to three titer steps compared with lots produced without IAC. The median anti-A and anti-B titers with IAC were 1:8 and 1:4, respectively, when measured by the Ph. Eur. direct method, and 1:2 and <1, respectively, when measured by the Ph. Eur. indirect method. The isoagglutinin flow cytometry assay showed an 87-90 % reduction in isoagglutinins in post-IAC versus pre-IAC fractions. IAC alone reduced anti-A and anti-B of the IgMs isotype by 92.5-97.8 % and 95.4-99.2 %, respectively. Other product quality characteristics were similar with and without IAC.
Conclusions: IAC is an effective method for reducing isoagglutinin levels in IVIG, and it is feasible on an industrial scale.
Conflict of interest statement
Compliance with Ethical Standards This article does not contain any new studies with human or animal subjects performed by any of the authors. Plasma donors gave written consent that their plasma may be used for research purposes. Funding This study was funded by CSL Behring AG. Medical writing assistance was provided by Vanessa Cobb and Angela Corstorphine of Kstorfin Medical Communications Ltd, supported by CSL Behring AG. Conflict of interest Simon Gerber, Annette Gaida, Nicole Spiegl, Sandra Wymann, Adriano Marques Antunes, Ibrahim El Menyawi, Brigitte Zurbriggen, Alphonse Hubsch, and Martin Imboden are employees of CSL Behring AG. Alphonse Hubsch, Brigitte Zurbriggen, and Martin Imboden own stocks in CSL Behring. Simon Gerber, Annette Gaida, Nicole Spiegl, Sandra Wymann, Adriano Marques Antunes, and Ibrahim El Menyawi have no further conflicts of interest. Author contributions Simon Gerber: process development of IAC step; compilation, analysis, and interpretation of data; manuscript drafting. Annette Gaida: development of IAC step; compilation, analysis, and interpretation of data; manuscript drafting. Sandra Wymann: development of isoagglutinin flow cytometry assays for IgG and IgM anti-A/B isoagglutinins; manuscript drafting. Ibrahim El Menyawi: process development of IAC step; generation of IgM anti-A/B isoagglutinins experimental data; manuscript drafting. Brigitte Zurbriggen: project management; manuscript drafting. Alphonse Hubsch: analysis and interpretation of data; manuscript drafting. Nicole Spiegl: support for development and implementation of the isoagglutinin flow cytometry assay in quality control; manuscript drafting. Adriano Marques Antunes: development and implementation of the isoagglutinin flow cytometry assay in quality control; manuscript drafting. Martin Imboden: process development for the IAC step; manuscript drafting.
Figures



References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

