The Mucus Barrier to Inhaled Gene Therapy
- PMID: 27646604
- PMCID: PMC5167788
- DOI: 10.1038/mt.2016.182
The Mucus Barrier to Inhaled Gene Therapy
Abstract
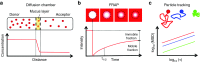
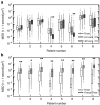
Recent evidence suggests that the airway mucus gel layer may be impermeable to the viral and synthetic gene vectors used in past inhaled gene therapy clinical trials for diseases like cystic fibrosis. These findings support the logic that inhaled gene vectors that are incapable of penetrating the mucus barrier are unlikely to provide meaningful benefit to patients. In this review, we discuss the biochemical and biophysical features of mucus that contribute its barrier function, and how these barrier properties may be reinforced in patients with lung disease. We next review biophysical techniques used to assess the potential ability of gene vectors to penetrate airway mucus. Finally, we provide new data suggesting that fresh human airway mucus should be used to test the penetration rates of gene vectors. The physiological barrier properties of spontaneously expectorated CF sputum remained intact up to 24 hours after collection when refrigerated at 4 °C. Conversely, the barrier properties were significantly altered after freezing and thawing of sputum samples. Gene vectors capable of overcoming the airway mucus barrier hold promise as a means to provide the widespread gene transfer throughout the airway epithelium required to achieve meaningful patient outcomes in inhaled gene therapy clinical trials.
Figures



References
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous

