Genetic Determinants of Cisplatin Resistance in Patients With Advanced Germ Cell Tumors
- PMID: 27646943
- PMCID: PMC5477828
- DOI: 10.1200/JCO.2016.68.7798
Genetic Determinants of Cisplatin Resistance in Patients With Advanced Germ Cell Tumors
Abstract
Purpose Owing to its exquisite chemotherapy sensitivity, most patients with metastatic germ cell tumors (GCTs) are cured with cisplatin-based chemotherapy. However, up to 30% of patients with advanced GCT exhibit cisplatin resistance, which requires intensive salvage treatment, and have a 50% risk of cancer-related death. To identify a genetic basis for cisplatin resistance, we performed whole-exome and targeted sequencing of cisplatin-sensitive and cisplatin-resistant GCTs. Methods Men with GCT who received a cisplatin-containing chemotherapy regimen and had available tumor tissue were eligible to participate in this study. Whole-exome sequencing or targeted exon-capture-based sequencing was performed on 180 tumors. Patients were categorized as cisplatin sensitive or cisplatin resistant by using a combination of postchemotherapy parameters, including serum tumor marker levels, radiology, and pathology at surgical resection of residual disease. Results TP53 alterations were present exclusively in cisplatin-resistant tumors and were particularly prevalent among primary mediastinal nonseminomas (72%). TP53 pathway alterations including MDM2 amplifications were more common among patients with adverse clinical features, categorized as poor risk according to the International Germ Cell Cancer Collaborative Group (IGCCCG) model. Despite this association, TP53 and MDM2 alterations predicted adverse prognosis independent of the IGCCCG model. Actionable alterations, including novel RAC1 mutations, were detected in 55% of cisplatin-resistant GCTs. Conclusion In GCT, TP53 and MDM2 alterations were associated with cisplatin resistance and inferior outcomes, independent of the IGCCCG model. The finding of frequent TP53 alterations among mediastinal primary nonseminomas may explain the more frequent chemoresistance observed with this tumor subtype. A substantial portion of cisplatin-resistant GCTs harbor actionable alterations, which might respond to targeted therapies. Genomic profiling of patients with advanced GCT could improve current risk stratification and identify novel therapeutic approaches for patients with cisplatin-resistant disease.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures
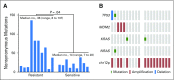



Comment in
-
Testicular cancer: Genetic determinants of cisplatin resistance.Nat Rev Urol. 2016 Nov;13(11):629. doi: 10.1038/nrurol.2016.197. Epub 2016 Oct 4. Nat Rev Urol. 2016. PMID: 27698397 No abstract available.
-
Re: Genetic Determinants of Cisplatin Resistance in Patients with Advanced Germ Cell Tumors.J Urol. 2017 Oct;198(4):746. doi: 10.1016/j.juro.2017.06.090. Epub 2017 Jul 3. J Urol. 2017. PMID: 28905790 No abstract available.
References
-
- Feldman DR, Bosl GJ, Sheinfeld J, et al. Medical treatment of advanced testicular cancer. JAMA. 2008;299:672–684. - PubMed
-
- Bosl GJ, Motzer RJ. Testicular germ-cell cancer. N Engl J Med. 1997;337:242–253. - PubMed
-
- Feldman DR, Iyer G, Van Alstine L, et al. Presence of somatic mutations within PIK3CA, AKT, RAS, and FGFR3 but not BRAF in cisplatin-resistant germ cell tumors. Clin Cancer Res. 2014;20:3712–3720. - PubMed
-
- Honecker F, Wermann H, Mayer F, et al. Microsatellite instability, mismatch repair deficiency, and BRAF mutation in treatment-resistant germ cell tumors. J Clin Oncol. 2009;27:2129–2136. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

