Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort
- PMID: 27646946
- PMCID: PMC6804896
- DOI: 10.1200/JCO.2016.68.1478
Antitumor Activity of Pembrolizumab in Biomarker-Unselected Patients With Recurrent and/or Metastatic Head and Neck Squamous Cell Carcinoma: Results From the Phase Ib KEYNOTE-012 Expansion Cohort
Abstract
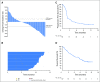
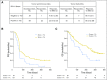
Purpose Treatment with pembrolizumab, an anti-programmed death-1 antibody, at 10 mg/kg administered once every 2 weeks, displayed durable antitumor activity in programmed death-ligand 1 (PD-L1) -positive recurrent and/or metastatic (R/M) head and neck squamous cell carcinoma (HNSCC) in the KEYNOTE-012 trial. Results from the expansion cohort, in which patients with HNSCC, irrespective of biomarker status, received a fixed dose of pembrolizumab at a less frequent dosing schedule, are reported. Patients and Methods Patients with R/M HNSCC, irrespective of PD-L1 or human papillomavirus status, received pembrolizumab 200 mg intravenously once every 3 weeks. Imaging was performed every 8 weeks. Primary end points were overall response rate (ORR) per central imaging vendor (Response Evaluation Criteria in Solid Tumors v1.1) and safety. Secondary end points included progression-free survival, overall survival, and association of response and PD-L1 expression. Patients who received one or more doses of pembrolizumab were included in analyses. Results Of 132 patients enrolled, median age was 60 years (range, 25 to 84 years), 83% were male, and 57% received two or more lines of therapy for R/M disease. ORR was 18% (95% CI, 12 to 26) by central imaging vendor and 20% (95% CI, 13 to 28) by investigator review. Median duration of response was not reached (range, ≥ 2 to ≥ 11 months). Six-month progression-free survival and overall survival rates were 23% and 59%, respectively. By using tumor and immune cells, a statistically significant increase in ORR was observed for PD-L1-positive versus -negative patients (22% v 4%; P = .021). Treatment-related adverse events of any grade and grade ≥ 3 events occurred in 62% and 9% of patients, respectively. Conclusion Fixed-dose pembrolizumab 200 mg administered once every 3 weeks was well tolerated and yielded a clinically meaningful ORR with evidence of durable responses, which supports further development of this regimen in patients with advanced HNSCC.
Trial registration: ClinicalTrials.gov NCT01848834.
Conflict of interest statement
Authors’ disclosures of potential conflicts of interest are found in the article online at
Figures
Comment in
-
Immune Checkpoint Inhibition in Cancers that Affect the Head and Neck.Int J Radiat Oncol Biol Phys. 2017 Aug 1;98(5):969-973. doi: 10.1016/j.ijrobp.2017.03.003. Epub 2017 Jul 10. Int J Radiat Oncol Biol Phys. 2017. PMID: 28721906 No abstract available.
References
-
- Ferlay J, Soerjomataram I, Dikshit R, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer. 2015;136:E359–E386. - PubMed
-
- Cohen EE, LaMonte SJ, Erb NL, et al. American Cancer Society head and neck cancer survivorship care guideline. CA Cancer J Clin. 2016;66:203–239. - PubMed
-
- Denaro N, Russi EG, Adamo V, et al. State-of-the-art and emerging treatment options in the management of head and neck cancer: News from 2013. Oncology. 2014;86:212–229. - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials

