Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation
- PMID: 27647168
- PMCID: PMC5293840
- DOI: 10.1136/heartjnl-2016-309832
Evolving antithrombotic treatment patterns for patients with newly diagnosed atrial fibrillation
Abstract
Objective: We studied evolving antithrombotic therapy patterns in patients with newly diagnosed non-valvular atrial fibrillation (AF) and ≥1 additional stroke risk factor between 2010 and 2015.
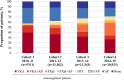
Methods: 39 670 patients were prospectively enrolled in four sequential cohorts in the Global Anticoagulant Registry in the FIELD-Atrial Fibrillation (GARFIELD-AF): cohort C1 (2010-2011), n=5500; C2 (2011-2013), n=11 662; C3 (2013-2014), n=11 462; C4 (2014-2015), n=11 046. Baseline characteristics and antithrombotic therapy initiated at diagnosis were analysed by cohort.
Results: Baseline characteristics were similar across cohorts. Median CHA2DS2-VASc (cardiac failure, hypertension, age ≥75 (doubled), diabetes, stroke (doubled)-vascular disease, age 65-74 and sex category (female)) score was 3 in all four cohorts. From C1 to C4, the proportion of patients on anticoagulant (AC) therapy increased by almost 15% (C1 57.4%; C4 71.1%). Use of vitamin K antagonist (VKA)±antiplatelet (AP) (C1 53.2%; C4 34.0%) and AP monotherapy (C1 30.2%; C4 16.6%) declined, while use of non-VKA oral ACs (NOACs)±AP increased (C1 4.2%; C4 37.0%). Most CHA2DS2-VASc ≥2 patients received AC, and this proportion increased over time, largely driven by NOAC prescribing. NOACs were more frequently prescribed than VKAs in men, the elderly, patients of Asian ethnicity, those with dementia, or those using non-steroidal anti-inflammatory drugs, and current smokers. VKA use was more common in patients with cardiac, vascular, or renal comorbidities.
Conclusions: Since NOACs were introduced, there has been an increase in newly diagnosed patients with AF at risk of stroke receiving guideline-recommended therapy, predominantly driven by increased use of NOACs and reduced use of VKA±AP or AP alone.
Trial registration number: NCT01090362; Pre-results.
Keywords: Stroke.
Published by the BMJ Publishing Group Limited. For permission to use (where not already granted under a licence) please go to http://www.bmj.com/company/products-services/rights-and-licensing/.
Conflict of interest statement
AJC: advisor to Bayer, Boehringer Ingelheim, Pfizer/BMS, and Daiichi Sankyo. GAm: advisor to Merck, Menarini, and Angelini. DA: personal fees from Bayer Healthcare, BMS/Pfizer, Boehringer-Ingelheim, and MSD. J-PB: personal fees from Aspen. FC: personal fees from Bayer, BMS, and Boehringer-Ingelheim. DAF: personal fees from BMS/Pfizer, Boehringer-Ingelheim, Daiichi Sankyo, and Bayer. SZG: grants from BiO2 Medical, Boehringer-Ingelheim, Bristol Meyers Squibb, BTG EKOS, Daiichi Sankyo, National Heart Lung and Blood Institute of the National Institutes of Health, Janssen, and Thrombosis Research Group; personal fees from Bayer, Boehringer-Ingelheim, Bristol Meyers Squibb, Daiichi Sankyo, Janssen, and Portola. SG: personal fees from the TRI, Bayer, and AstraZeneca; grants from Sanofi and Pfizer. SH: personal fees from Aspen, Bayer Healthcare, BMS/Pfizer, Daiichi-Sankyo, and Sanofi. YK: grants and personal fees from Daiichi Sankyo and Boehringer-Ingelheim; personal fees from Bayer, Bristol-Meyers Squibb, and Pfizer. LGM: grants and personal fees from Bayer Healthcare and Pfizer; grants from Boehringer Ingelheim; personal fees from Daiichi Sankyo. FM: employee of Bayer Pharma AG. SO: consultant/advisory board payments from Bayer Pharma AG, Bristol-Myers Squibb Korea, Boehringer-Ingelheim Korea, Pfizer Korea, Sanofi-Aventis, and St Jude Medical. AGGT: personal fees from Bayer Healthcare, Janssen Pharmaceutical Research & Development LLC, Astellas, Portola, and Takeda. FWAV: personal fees from Bayer Healthcare, Daiichi-Sankyo, BMS/Pfizer, and Boehringer-Ingelheim. AKK: grants and personal fees from Bayer Healthcare; personal fees from Boehringer-Ingelheim Pharma, Daiichi Sankyo Europe, Sanofi SA, Janssen.
Figures


References
-
- Camm AJ, Lip GY, De Caterina R, et al. . 2012 focused update of the ESC Guidelines for the management of atrial fibrillation: an update of the 2010 ESC Guidelines for the management of atrial fibrillation. Developed with the special contribution of the European Heart Rhythm Association. Eur Heart J 2012;33:2719–47. 10.1093/eurheartj/ehs253 - DOI - PubMed
-
- January CT, Wann LS, Alpert JS, et al. . 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines and the Heart Rhythm Society. Circulation 2014;130:e199–267. 10.1161/CIR.0000000000000041 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
