Multi-Phenotypic subtyping of circulating tumor cells using sequential fluorescent quenching and restaining
- PMID: 27647345
- PMCID: PMC5028835
- DOI: 10.1038/srep33488
Multi-Phenotypic subtyping of circulating tumor cells using sequential fluorescent quenching and restaining
Abstract
In tissue biopsies formalin fixed paraffin embedded cancer blocks are micro-sectioned producing multiple semi-identical specimens which are analyzed and subtyped proteomically, and genomically, with numerous biomarkers. In blood based biopsies (BBBs), blood is purified for circulating tumor cells (CTCs) and clinical utility is typically limited to cell enumeration, as only 2-3 positive fluorescent markers and 1 negative marker can be used. As such, increasing the number of subtyping biomarkers on each individual CTC could dramatically enhance the clinical utility of BBBs, allowing in depth interrogation of clinically relevant CTCs. We describe a simple and inexpensive method for quenching the specific fluors of fluorescently stained CTCs followed by sequential restaining with additional biomarkers. As proof of principle a CTC panel, immunosuppression panel and stem cell panel were used to sequentially subtype individual fluorescently stained patient CTCs, suggesting a simple and universal technique to analyze multiple clinically applicable immunomarkers from BBBs.
Conflict of interest statement
D. Adams and CM. Tang are employees at Creatv Microtech, Inc. S. Stefansson is an employee of HeMemics Biotechnologies, Inc.
Figures
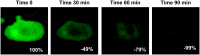
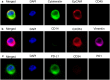


References
-
- Adams D. L. et al. Cytometric characterization of circulating tumor cells captured by microfiltration and their correlation to the cellsearch((R)) CTC test. Cytometry. Part A: the journal of the International Society for Analytical Cytology 87, 137–144, doi: 10.1002/cyto.a.22613 (2015). - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

