Long-chain acyl-CoA synthetase 6 regulates lipid synthesis and mitochondrial oxidative capacity in human and rat skeletal muscle
- PMID: 27647415
- PMCID: PMC5285616
- DOI: 10.1113/JP272962
Long-chain acyl-CoA synthetase 6 regulates lipid synthesis and mitochondrial oxidative capacity in human and rat skeletal muscle
Abstract
Key points: Long-chain acyl-CoA synthetase 6 (ACSL6) mRNA is present in human and rat skeletal muscle, and is modulated by nutritional status: exercise and fasting decrease ACSL6 mRNA, whereas acute lipid ingestion increase its expression. ACSL6 genic inhibition in rat primary myotubes decreased lipid accumulation, as well as activated the higher mitochondrial oxidative capacity programme and fatty acid oxidation through the AMPK/PGC1-α pathway. ACSL6 overexpression in human primary myotubes increased phospholipid species and decreased oxidative metabolism.
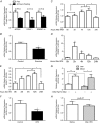
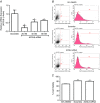
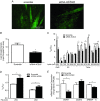
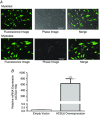
Abstract: Long-chain acyl-CoA synthetases (ACSL 1 to 6) are key enzymes regulating the partitioning of acyl-CoA species toward different metabolic fates such as lipid synthesis or β-oxidation. Despite our understanding of ecotopic lipid accumulation in skeletal muscle being associated with metabolic diseases such as obesity and type II diabetes, the role of specific ACSL isoforms in lipid synthesis remains unclear. In the present study, we describe for the first time the presence of ACSL6 mRNA in human skeletal muscle and the role that ACSL6 plays in lipid synthesis in both rodent and human skeletal muscle. ACSL6 mRNA was observed to be up-regulated by acute high-fat meal ingestion in both rodents and humans. In rats, we also demonstrated that fasting and chronic aerobic training negatively modulated the ACSL6 mRNA and other genes of lipid synthesis. Similar results were obtained following ACSL6 knockdown in rat myotubes, which was associated with a decreased accumulation of TAGs and lipid droplets. Under the same knockdown condition, we further demonstrate an increase in fatty acid content, p-AMPK, mitochondrial content, mitochondrial respiratory rates and palmitate oxidation. These results were associated with increased PGC-1α, UCP2 and UCP3 mRNA and decreased reactive oxygen species production. In human myotubes, ACSL6 overexpression reduced palmitate oxidation and PGC-1α mRNA. In conclusion, ACSL6 drives acyl-CoA toward lipid synthesis and its downregulation improves mitochondrial biogenesis, respiratory capacity and lipid oxidation. These outcomes are associated with the activation of the AMPK/PGC1-α pathway.
Keywords: ACSL6; human skeletal muscle; long-chain acyl-CoA synthetase; mitochondria; primary skeletal muscle cells; triacylglycerol synthesis; β-oxidation.
© 2016 The Authors. The Journal of Physiology © 2016 The Physiological Society.
Figures



Comment in
-
Is ACSL6 at the crossroads of skeletal muscle lipid synthesis?J Physiol. 2017 Feb 1;595(3):619-620. doi: 10.1113/JP273460. J Physiol. 2017. PMID: 28145005 Free PMC article. No abstract available.
Similar articles
-
Diet and Exercise Training Influence Skeletal Muscle Long-Chain acyl-CoA Synthetases.Med Sci Sports Exerc. 2020 Mar;52(3):569-576. doi: 10.1249/MSS.0000000000002164. Med Sci Sports Exerc. 2020. PMID: 31524824 Free PMC article.
-
Suppression of long chain acyl-CoA synthetase blocks intracellular fatty acid flux and glucose uptake in skeletal myotubes.Biochim Biophys Acta Mol Cell Biol Lipids. 2020 Jul;1865(7):158678. doi: 10.1016/j.bbalip.2020.158678. Epub 2020 Feb 29. Biochim Biophys Acta Mol Cell Biol Lipids. 2020. PMID: 32126286
-
Overexpression of Long-Chain Acyl-CoA Synthetase 5 Increases Fatty Acid Oxidation and Free Radical Formation While Attenuating Insulin Signaling in Primary Human Skeletal Myotubes.Int J Environ Res Public Health. 2019 Mar 31;16(7):1157. doi: 10.3390/ijerph16071157. Int J Environ Res Public Health. 2019. PMID: 30935113 Free PMC article.
-
Regulation of energy metabolism by long-chain fatty acids.Prog Lipid Res. 2014 Jan;53:124-44. doi: 10.1016/j.plipres.2013.12.001. Epub 2013 Dec 18. Prog Lipid Res. 2014. PMID: 24362249 Review.
-
Peroxisomal acyl-CoA synthetases.Biochim Biophys Acta. 2012 Sep;1822(9):1411-20. doi: 10.1016/j.bbadis.2012.02.010. Epub 2012 Feb 17. Biochim Biophys Acta. 2012. PMID: 22366061 Free PMC article. Review.
Cited by
-
Diet and Exercise Training Influence Skeletal Muscle Long-Chain acyl-CoA Synthetases.Med Sci Sports Exerc. 2020 Mar;52(3):569-576. doi: 10.1249/MSS.0000000000002164. Med Sci Sports Exerc. 2020. PMID: 31524824 Free PMC article.
-
Manipulation of Host Cholesterol by Obligate Intracellular Bacteria.Front Cell Infect Microbiol. 2017 May 5;7:165. doi: 10.3389/fcimb.2017.00165. eCollection 2017. Front Cell Infect Microbiol. 2017. PMID: 28529926 Free PMC article. Review.
-
Molecular Characterization, Tissue Distribution Profile, and Nutritional Regulation of acsl Gene Family in Golden Pompano (Trachinotus ovatus).Int J Mol Sci. 2022 Jun 9;23(12):6437. doi: 10.3390/ijms23126437. Int J Mol Sci. 2022. PMID: 35742881 Free PMC article.
-
Description of an Institutional Cohort of Myeloid Neoplasms Carrying ETV6-Locus Deletions or ETV6 Rearrangements.Acta Haematol. 2023;146(5):401-407. doi: 10.1159/000529844. Epub 2023 Feb 27. Acta Haematol. 2023. PMID: 36848872 Free PMC article.
-
Transcriptomic and Metabolomic Analyses Reveal Inhibition of Hepatic Adipogenesis and Fat Catabolism in Yak for Adaptation to Forage Shortage During Cold Season.Front Cell Dev Biol. 2022 Jan 17;9:759521. doi: 10.3389/fcell.2021.759521. eCollection 2021. Front Cell Dev Biol. 2022. PMID: 35111749 Free PMC article.
References
-
- Adamo KB, Dent R, Langefeld CD, Cox M, Williams K, Carrick KM, Stuart JS, Sundseth SS, Harper ME, McPherson R & Tesson F (2007). Peroxisome proliferator‐activated receptor gamma 2 and acyl‐CoA synthetase 5 polymorphisms influence diet response. Obesity (Silver Spring) 15, 1068–1075. - PubMed
-
- Alberici LC, Oliveira HC, Catharino RR, Vercesi AE, Eberlin MN & Alberici RM (2011). Distinct hepatic lipid profile of hypertriglyceridemic mice determined by easy ambient sonic‐spray ionization mass spectrometry. Anal Bioanal Chem 401, 1651–1659. - PubMed
-
- Anderson EJ, Lustig ME, Boyle KE, Woodlief TL, Kane DA, Lin CT, Price JW, Kang L, Rabinovitch PS, Szeto HH, Houmard JA, Cortright RN, Wasserman DH & Neufer PD (2009). Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. J Clin Invest 119, 573–578. - PMC - PubMed
-
- Bizeau ME, MacLean PS, Johnson GC & Wei Y (2003). Skeletal muscle sterol regulatory element binding protein‐1c decreases with food deprivation and increases with feeding in rats. J Nutr 133, 1787–1792. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

