PCL-PEG-PCL film promotes cartilage regeneration in vivo
- PMID: 27647680
- PMCID: PMC6496874
- DOI: 10.1111/cpr.12295
PCL-PEG-PCL film promotes cartilage regeneration in vivo
Abstract
Objective: Management of chondral defects has long been a challenge due to poor self-healing capacity of articular cartilage. Many approaches, ranging from symptomatic treatment to structural cartilage regeneration, have obtained very limited satisfactory results. Cartilage tissue engineering, which involves optimized combination of novel scaffolds, cell sources and growth factors, has emerged as a promising strategy for cartilage regeneration and repair. In this study, the aim was to investigate the role of poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) (PCL-PEG-PCL, PCEC) PCEC scaffold in cartilage repair.
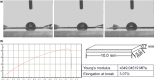
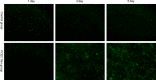
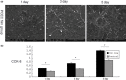
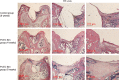
Materials and methods: First, PCEC film was fabricated, and its characteristics were tested using SEM and AFM. Cell (rASC - rat adipose-derived stem cells, and mASCs - green fluorescent mouse adipose-derived stem cells) morphologies on PCEC film were observed using SEM and fluorescence microscopy, after cell seeding. Tests of cell viability on PCEC film were conducted using the CCK-8 assay. Furthermore, full cartilage defects in rats were created, and PCEC films were implanted, to evaluate their healing effects, over 8 weeks.
Results: It was found that PCEC film, as a biomaterial implant, possessed good in vitro properties for cell adhesion, migration and proliferation. Importantly, in the in vivo experiment, PCEC film exhibited desirable healing outcomes.
Conclusions: These results demonstrated that PCEC film was a good scaffold for cartilage tissue engineering for improving cell proliferation and adhesion and could lead to excellent repair of cartilage defects.
© 2016 John Wiley & Sons Ltd.
Figures



Similar articles
-
Acceleration of dermal wound healing by using electrospun curcumin-loaded poly(ε-caprolactone)-poly(ethylene glycol)-poly(ε-caprolactone) fibrous mats.J Biomed Mater Res B Appl Biomater. 2014 Apr;102(3):533-42. doi: 10.1002/jbm.b.33032. Epub 2013 Sep 20. J Biomed Mater Res B Appl Biomater. 2014. PMID: 24115465
-
Chondrocyte Co-cultures with the Stromal Vascular Fraction of Adipose Tissue in Polyhydroxybutyrate/Poly-(hydroxybutyrate-co-hydroxyhexanoate) Scaffolds: Evaluation of Cartilage Repair in Rabbit.Cell Transplant. 2019 Nov;28(11):1432-1438. doi: 10.1177/0963689719861275. Epub 2019 Jul 24. Cell Transplant. 2019. PMID: 31337228 Free PMC article.
-
Synthesis and in vitro evaluation of thermosensitive hydrogel scaffolds based on (PNIPAAm-PCL-PEG-PCL-PNIPAAm)/Gelatin and (PCL-PEG-PCL)/Gelatin for use in cartilage tissue engineering.J Biomater Sci Polym Ed. 2018 Jul;29(10):1185-1206. doi: 10.1080/09205063.2018.1447627. Epub 2018 Mar 5. J Biomater Sci Polym Ed. 2018. PMID: 29490569
-
Strategies for improving the repair of focal cartilage defects.Nanomedicine (Lond). 2015;10(18):2893-905. doi: 10.2217/nnm.15.119. Epub 2015 Sep 7. Nanomedicine (Lond). 2015. PMID: 26377158 Review.
-
Tissue Engineering: An Alternative to Repair Cartilage.Tissue Eng Part B Rev. 2019 Aug;25(4):357-373. doi: 10.1089/ten.TEB.2018.0330. Tissue Eng Part B Rev. 2019. PMID: 30913997 Review.
Cited by
-
Applications of Biocompatible Scaffold Materials in Stem Cell-Based Cartilage Tissue Engineering.Front Bioeng Biotechnol. 2021 Mar 25;9:603444. doi: 10.3389/fbioe.2021.603444. eCollection 2021. Front Bioeng Biotechnol. 2021. PMID: 33842441 Free PMC article. Review.
-
Collagen-derived dipeptide prolyl-hydroxyproline promotes osteogenic differentiation through Foxg1.Cell Mol Biol Lett. 2017 Dec 1;22:27. doi: 10.1186/s11658-017-0060-2. eCollection 2017. Cell Mol Biol Lett. 2017. PMID: 29213293 Free PMC article.
-
Production of Plant-Based, Film-Type Scaffolds Using Alginate and Corn Starch for the Culture of Bovine Myoblasts.Foods. 2024 Apr 28;13(9):1358. doi: 10.3390/foods13091358. Foods. 2024. PMID: 38731729 Free PMC article.
-
MMP-2 and Notch signal pathway regulate migration of adipose-derived stem cells and chondrocytes in co-culture systems.Cell Prolif. 2017 Dec;50(6):e12385. doi: 10.1111/cpr.12385. Epub 2017 Sep 18. Cell Prolif. 2017. PMID: 28925018 Free PMC article.
-
Curved microstructures promote osteogenesis of mesenchymal stem cells via the RhoA/ROCK pathway.Cell Prolif. 2017 Aug;50(4):e12356. doi: 10.1111/cpr.12356. Cell Prolif. 2017. PMID: 28714177 Free PMC article.
References
-
- Kuettner KE, Cole AA. Cartilage degeneration in different human joints. Osteoarthritis Cartilage. 2005;13:93–103. - PubMed
-
- Jackson DW, Simon TM. Chondrocyte transplantation. Arthroscopy. 1996;12:732–738. - PubMed
-
- Newman AP. Articular cartilage repair. Am J Sports Med. 1998;26:309–324. - PubMed
-
- Bert JM. Role of abrasion arthroplasty and debridement in the management of osteoarthritis of the knee. Rheum Dis Clin North Am. 1993;19:725–739. - PubMed
-
- Johnson LL. Arthroscopic abrasion arthroplasty historical and pathologic perspective: present status. Arthroscopy. 1986;2:54–69. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

