Resistance to citrus canker induced by a variant of Xanthomonas citri ssp. citri is associated with a hypersensitive cell death response involving autophagy-associated vacuolar processes
- PMID: 27647752
- PMCID: PMC6638218
- DOI: 10.1111/mpp.12489
Resistance to citrus canker induced by a variant of Xanthomonas citri ssp. citri is associated with a hypersensitive cell death response involving autophagy-associated vacuolar processes
Abstract
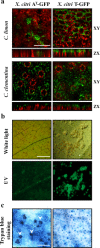
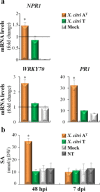
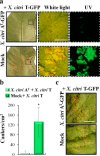
Xanthomonas citri ssp. citri (X. citri) is the causal agent of Asiatic citrus canker, a disease that seriously affects most commercially important Citrus species worldwide. We have identified previously a natural variant, X. citri AT , that triggers a host-specific defence response in Citrus limon. However, the mechanisms involved in this canker disease resistance are unknown. In this work, the defence response induced by X. citri AT was assessed by transcriptomic, physiological and ultrastructural analyses, and the effects on bacterial biofilm formation were monitored in parallel. We show that X. citri AT triggers a hypersensitive response associated with the interference of biofilm development and arrest of bacterial growth in C. limon. This plant response involves an extensive transcriptional reprogramming, setting in motion cell wall reinforcement, the oxidative burst and the accumulation of salicylic acid (SA) and phenolic compounds. Ultrastructural analyses revealed subcellular changes involving the activation of autophagy-associated vacuolar processes. Our findings show the activation of SA-dependent defence in response to X. citri AT and suggest a coordinated regulation between the SA and flavonoid pathways, which is associated with autophagy mechanisms that control pathogen invasion in C. limon. Furthermore, this defence response protects C. limon plants from disease on subsequent challenges by pathogenic X. citri. This knowledge will allow the rational exploitation of the plant immune system as a biotechnological approach for the management of the disease.
Keywords: autophagy; biofilm formation; biological control; citrus canker resistance; hypersensitive response; salicylic acid; secondary metabolites.
© 2016 BSPP AND JOHN WILEY & SONS LTD.
Figures



Similar articles
-
PthA4AT , a 7.5-repeats transcription activator-like (TAL) effector from Xanthomonas citri ssp. citri, triggers citrus canker resistance.Mol Plant Pathol. 2019 Oct;20(10):1394-1407. doi: 10.1111/mpp.12844. Epub 2019 Jul 5. Mol Plant Pathol. 2019. PMID: 31274237 Free PMC article.
-
Plant responses underlying nonhost resistance of Citrus limon against Xanthomonas campestris pv. campestris.Mol Plant Pathol. 2019 Feb;20(2):254-269. doi: 10.1111/mpp.12752. Epub 2018 Oct 31. Mol Plant Pathol. 2019. PMID: 30260546 Free PMC article.
-
Responsiveness of different citrus genotypes to the Xanthomonas citri ssp. citri-derived pathogen-associated molecular pattern (PAMP) flg22 correlates with resistance to citrus canker.Mol Plant Pathol. 2015 Jun;16(5):507-20. doi: 10.1111/mpp.12206. Epub 2014 Oct 27. Mol Plant Pathol. 2015. PMID: 25231217 Free PMC article.
-
XacFhaB adhesin, an important Xanthomonas citri ssp. citri virulence factor, is recognized as a pathogen-associated molecular pattern.Mol Plant Pathol. 2016 Dec;17(9):1344-1353. doi: 10.1111/mpp.12364. Epub 2016 Jun 9. Mol Plant Pathol. 2016. PMID: 26724481 Free PMC article.
-
Genetic and structural determinants on iron assimilation pathways in the plant pathogen Xanthomonas citri subsp. citri and Xanthomonas sp.Braz J Microbiol. 2020 Sep;51(3):1219-1231. doi: 10.1007/s42770-019-00207-x. Epub 2020 Aug 28. Braz J Microbiol. 2020. PMID: 31848911 Free PMC article. Review.
Cited by
-
PthA4AT , a 7.5-repeats transcription activator-like (TAL) effector from Xanthomonas citri ssp. citri, triggers citrus canker resistance.Mol Plant Pathol. 2019 Oct;20(10):1394-1407. doi: 10.1111/mpp.12844. Epub 2019 Jul 5. Mol Plant Pathol. 2019. PMID: 31274237 Free PMC article.
-
Transcriptome-wide identification and characterization of microRNAs in diverse phases of wood formation in Populus trichocarpa.G3 (Bethesda). 2021 Aug 7;11(8):jkab195. doi: 10.1093/g3journal/jkab195. G3 (Bethesda). 2021. PMID: 34849817 Free PMC article.
-
Botanical-Based Strategies for Controlling Xanthomonas spp. in Cotton and Citrus: In Vitro and In Vivo Evaluation.Plants (Basel). 2025 Mar 19;14(6):957. doi: 10.3390/plants14060957. Plants (Basel). 2025. PMID: 40265902 Free PMC article.
-
Plant responses underlying nonhost resistance of Citrus limon against Xanthomonas campestris pv. campestris.Mol Plant Pathol. 2019 Feb;20(2):254-269. doi: 10.1111/mpp.12752. Epub 2018 Oct 31. Mol Plant Pathol. 2019. PMID: 30260546 Free PMC article.
-
Structure-function relationship of a citrus salicylate methylesterase and role of salicylic acid in citrus canker resistance.Sci Rep. 2019 Mar 7;9(1):3901. doi: 10.1038/s41598-019-40552-3. Sci Rep. 2019. PMID: 30846791 Free PMC article.
References
-
- Bilgin, D.D. , Zavala, J.A. , Zhu, J. , Clough, S.J. , Ort, D.R. and De Lucia, E.H. (2010) Biotic stress globally downregulates photosynthesis genes. Plant Cell Environ. 33, 1597–1613. - PubMed
-
- Castillo, P. , Escalante, M. , Gallardo, M. , Alemano, S. and Abdala, G. (2013) Effects of bacterial single inoculation and co‐inoculation on growth and phytohormone production of sunflower seedlings under water stress. Acta Physiol. Plant. 35, 2299–2309.
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

