Direct IL-6 Signals Maximize Protective Secondary CD4 T Cell Responses against Influenza
- PMID: 27647834
- PMCID: PMC5101150
- DOI: 10.4049/jimmunol.1600033
Direct IL-6 Signals Maximize Protective Secondary CD4 T Cell Responses against Influenza
Abstract
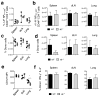
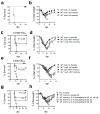
Memory T cells can often respond against pathogens that have evaded neutralizing Abs and are thus key to vaccine-induced protection, yet the signals needed to optimize their responses are unclear. In this study, we identify a dramatic and selective requirement for IL-6 to achieve optimal memory CD4 T cell recall following heterosubtypic influenza A virus (IAV) challenge of mice primed previously with wild-type or attenuated IAV strains. Through analysis of endogenous T cell responses and adoptive transfer of IAV-specific memory T cell populations, we find that without IL-6, CD4+, but not CD8+, secondary effector populations expand less and have blunted function and antiviral impact. Early and direct IL-6 signals to memory CD4 T cells are required to program maximal secondary effector responses at the site of infection during heterosubtypic challenge, indicating a novel role for a costimulatory cytokine in recall responses.
Copyright © 2016 by The American Association of Immunologists, Inc.
Figures

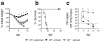




References
-
- Tan AC, Mifsud EJ, Zeng W, Edenborough K, McVernon J, Brown LE, Jackson DC. Intranasal administration of the TLR2 agonist Pam2Cys provides rapid protection against influenza in mice. Mol Pharm. 2012;9:2710–2718. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

