Crystal structures and dynamical properties of dense CO2
- PMID: 27647887
- PMCID: PMC5056093
- DOI: 10.1073/pnas.1601254113
Crystal structures and dynamical properties of dense CO2
Abstract
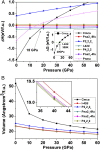
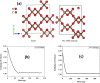
Structural polymorphism in dense carbon dioxide (CO2) has attracted significant attention in high-pressure physics and chemistry for the past two decades. Here, we have performed high-pressure experiments and first-principles theoretical calculations to investigate the stability, structure, and dynamical properties of dense CO2 We found evidence that CO2-V with the 4-coordinated extended structure can be quenched to ambient pressure below 200 K-the melting temperature of CO2-I. CO2-V is a fully coordinated structure formed from a molecular solid at high pressure and recovered at ambient pressure. Apart from confirming the metastability of CO2-V (I-42d) at ambient pressure at low temperature, results of ab initio molecular dynamics and metadynamics (MD) simulations provided insights into the transformation processes and structural relationship from the molecular to the extended phases. In addition, the simulation also predicted a phase V'(Pna21) in the stability region of CO2-V with a diffraction pattern similar to that previously assigned to the CO2-V (P212121) structure. Both CO2-V and -V' are predicted to be recoverable and hard with a Vicker hardness of ∼20 GPa. Significantly, MD simulations found that the CO2 in phase IV exhibits large-amplitude bending motions at finite temperatures and high pressures. This finding helps to explain the discrepancy between earlier predicted static structures and experiments. MD simulations clearly indicate temperature effects are critical to understanding the high-pressure behaviors of dense CO2 structures-highlighting the significance of chemical kinetics associated with the transformations.
Keywords: carbon dioxide; high pressure; material science; molecular dynamics.
Conflict of interest statement
The authors declare no conflict of interest.
Figures







Comment in
-
Reply to Datchi et al.: Recovered phase CO2-V at low temperature and a newly predicted 3D-extended CO2 phase.Proc Natl Acad Sci U S A. 2017 Jan 31;114(5):E658-E659. doi: 10.1073/pnas.1620267114. Epub 2017 Jan 17. Proc Natl Acad Sci U S A. 2017. PMID: 28096369 Free PMC article. No abstract available.
-
Polymeric phase V of carbon dioxide has not been recovered at ambient pressure and has a unique structure.Proc Natl Acad Sci U S A. 2017 Jan 31;114(5):E656-E657. doi: 10.1073/pnas.1619276114. Epub 2017 Jan 17. Proc Natl Acad Sci U S A. 2017. PMID: 28096370 Free PMC article. No abstract available.
References
-
- Santoro M, Lin JF, Mao HK, Hemley RJ. In situ high P-T Raman spectroscopy and laser heating of carbon dioxide. J Chem Phys. 2004;121(6):2780–2787. - PubMed
-
- Tschauner O, Mao HK, Hemley RJ. New transformations of CO(2) at high pressures and temperatures. Phys Rev Lett. 2001;87(7):075701. - PubMed
-
- Yoo C-S. Physical and chemical transformations of highly compressed carbon dioxide at bond energies. Phys Chem Chem Phys. 2013;15(21):7949–7966. - PubMed
-
- Santoro M, Gorelli FA. High pressure solid state chemistry of carbon dioxide. Chem Soc Rev. 2006;35(10):918–931. - PubMed
-
- Aoki K, Yamawaki H, Sakashita M, Gotoh Y, Takemura K. Crystal structure of the high-pressure phase of solid CO2. Science. 1994;263(5145):356–358. - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

