Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact
- PMID: 27647907
- PMCID: PMC5056099
- DOI: 10.1073/pnas.1613225113
Optimal activation of Fc-mediated effector functions by influenza virus hemagglutinin antibodies requires two points of contact
Abstract
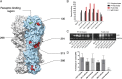
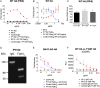
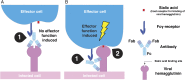
Influenza virus strain-specific monoclonal antibodies (mAbs) provide protection independent of Fc gamma receptor (FcγR) engagement. In contrast, optimal in vivo protection achieved by broadly reactive mAbs requires Fc-FcγR engagement. Most strain-specific mAbs target the head domain of the viral hemagglutinin (HA), whereas broadly reactive mAbs typically recognize epitopes within the HA stalk. This observation has led to questions regarding the mechanism regulating the activation of Fc-dependent effector functions by broadly reactive antibodies. To dissect the molecular mechanism responsible for this dichotomy, we inserted the FLAG epitope into discrete locations on HAs. By characterizing the interactions of several FLAG-tagged HAs with a FLAG-specific antibody, we show that in addition to Fc-FcγR engagement mediated by the FLAG-specific antibody, a second intermolecular bridge between the receptor-binding region of the HA and sialic acid on effector cells is required for optimal activation. Inhibition of this second molecular bridge, through the use of an F(ab')2 or the mutation of the sialic acid-binding site, renders the Fc-FcγR interaction unable to optimally activate effector cells. Our findings indicate that broadly reactive mAbs require two molecular contacts to possibly stabilize the immunologic synapse and potently induce antibody-dependent cell-mediated antiviral responses: (i) the interaction between the Fc of a mAb bound to HA with the FcγR of the effector cell and (ii) the interaction between the HA and its sialic acid receptor on the effector cell. This concept might be broadly applicable for protective antibody responses to viral pathogens that have suitable receptors on effector cells.
Keywords: Fcγ receptor; antibody-dependent cell-mediated immunity; broadly reactive antibodies; hemagglutinin; influenza virus.
Conflict of interest statement
The authors declare no conflict of interest.
Figures

Similar articles
-
Broadly Neutralizing Hemagglutinin Stalk-Specific Antibodies Induce Potent Phagocytosis of Immune Complexes by Neutrophils in an Fc-Dependent Manner.mBio. 2016 Oct 4;7(5):e01624-16. doi: 10.1128/mBio.01624-16. mBio. 2016. PMID: 27703076 Free PMC article.
-
Divergent Requirement of Fc-Fcγ Receptor Interactions for In Vivo Protection against Influenza Viruses by Two Pan-H5 Hemagglutinin Antibodies.J Virol. 2017 May 12;91(11):e02065-16. doi: 10.1128/JVI.02065-16. Print 2017 Jun 1. J Virol. 2017. PMID: 28331095 Free PMC article.
-
Epitope specificity plays a critical role in regulating antibody-dependent cell-mediated cytotoxicity against influenza A virus.Proc Natl Acad Sci U S A. 2016 Oct 18;113(42):11931-11936. doi: 10.1073/pnas.1609316113. Epub 2016 Oct 3. Proc Natl Acad Sci U S A. 2016. PMID: 27698132 Free PMC article.
-
Fc or not Fc; that is the question: Antibody Fc-receptor interactions are key to universal influenza vaccine design.Hum Vaccin Immunother. 2017 Jun 3;13(6):1-9. doi: 10.1080/21645515.2017.1290018. Epub 2017 Mar 23. Hum Vaccin Immunother. 2017. PMID: 28332900 Free PMC article. Review.
-
Extra-Neutralizing FcR-Mediated Antibody Functions for a Universal Influenza Vaccine.Front Immunol. 2019 Mar 18;10:440. doi: 10.3389/fimmu.2019.00440. eCollection 2019. Front Immunol. 2019. PMID: 30949165 Free PMC article. Review.
Cited by
-
Influenza Virus Infection Enhances Antibody-Mediated NK Cell Functions via Type I Interferon-Dependent Pathways.J Virol. 2019 Feb 19;93(5):e02090-18. doi: 10.1128/JVI.02090-18. Print 2019 Mar 1. J Virol. 2019. PMID: 30541850 Free PMC article.
-
Influenza Anti-Stalk Antibodies: Development of a New Method for the Evaluation of the Immune Responses to Universal Vaccine.Vaccines (Basel). 2020 Jan 24;8(1):43. doi: 10.3390/vaccines8010043. Vaccines (Basel). 2020. PMID: 31991681 Free PMC article.
-
A Live-Attenuated Prime, Inactivated Boost Vaccination Strategy with Chimeric Hemagglutinin-Based Universal Influenza Virus Vaccines Provides Protection in Ferrets: A Confirmatory Study.Vaccines (Basel). 2018 Jul 25;6(3):47. doi: 10.3390/vaccines6030047. Vaccines (Basel). 2018. PMID: 30044403 Free PMC article.
-
Antibody Responses toward the Major Antigenic Sites of Influenza B Virus Hemagglutinin in Mice, Ferrets, and Humans.J Virol. 2019 Jan 4;93(2):e01673-18. doi: 10.1128/JVI.01673-18. Print 2019 Jan 15. J Virol. 2019. PMID: 30381487 Free PMC article.
-
Differential requirements for FcγR engagement by protective antibodies against Ebola virus.Proc Natl Acad Sci U S A. 2019 Oct 1;116(40):20054-20062. doi: 10.1073/pnas.1911842116. Epub 2019 Sep 4. Proc Natl Acad Sci U S A. 2019. PMID: 31484758 Free PMC article.
References
-
- World Health Organization 2014 Fact Sheet 211: Influenza. Available at: www.who.int/mediacentre/factsheets/fs211/en/. Accessed September 9, 2016.
-
- Krammer F, Palese P, Steel J. Advances in universal influenza virus vaccine design and antibody-mediated therapies based on conserved regions of the hemagglutinin. Curr Top Microbiol Immunol. 2014;386:301–321. - PubMed
-
- Shaw ML, Palese P. Orthomyxoviruses. In: Knipe DM, Howley PM, editors. Fields Virology. 4th Ed Lippincott-Raven; Philadelphia, PA: 2007.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

