Identification of keratan sulfate disaccharide at C-3 position of glucuronate of chondroitin sulfate from Mactra chinensis
- PMID: 27647934
- PMCID: PMC5103875
- DOI: 10.1042/BCJ20160655
Identification of keratan sulfate disaccharide at C-3 position of glucuronate of chondroitin sulfate from Mactra chinensis
Abstract
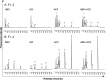
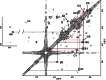
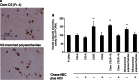
Glycosaminoglycans (GAGs), including chondroitin sulfate (CS), dermatan sulfate, heparin, heparan sulfate and keratan sulfate (KS) are linear sulfated repeating disaccharide sequences containing hexosamine and uronic acid [or galactose (Gal) in the case of KS]. Among the GAGs, CS shows structural variations, such as sulfation patterns and fucosylation, which are responsible for their physiological functions through CS interaction with CS-binding proteins. Here, we solved the structure of KS-branched CS-E derived from a clam, Mactra chinensis KS disaccharide [d-GlcNAc6S-(1→3)-β-d-Gal-(1→] was attached to the C-3 position of GlcA, and consecutive KS-branched disaccharide sequences were found in a CS chain. KS-branched polysaccharides clearly exhibited resistance to degradation by chondroitinase ABC or ACII (at low concentrations) compared with typical CS structures. Furthermore, KS-branched polysaccharides stimulated neurite outgrowth of hippocampal neurons. These results strongly suggest that M. chinensis is a rich source of KS-branched CS, and it has important biological activities.
Keywords: Glycobiology; chondroitin sulfate; chondroitinase; keratan sulfate; proteoglycan.
© 2016 The Author(s).
Figures




Similar articles
-
Isolation of Keratan Sulfate Disaccharide-branched Chondroitin Sulfate E from Mactra chinensis.Bio Protoc. 2017 Aug 5;7(15):e2441. doi: 10.21769/BioProtoc.2441. eCollection 2017 Aug 5. Bio Protoc. 2017. PMID: 34541160 Free PMC article.
-
Preparation of the Partially Methylated Alditol Acetates Derived from CS Tetrasaccharides Containing Galactose for the Gas Chromatography/Mass Spectrometry Analysis.Bio Protoc. 2017 Nov 5;7(21):e2600. doi: 10.21769/BioProtoc.2600. eCollection 2017 Nov 5. Bio Protoc. 2017. PMID: 34595277 Free PMC article.
-
Comprehensive analysis of glycosaminoglycans from the edible shellfish.Carbohydr Polym. 2018 Mar 15;184:269-276. doi: 10.1016/j.carbpol.2017.12.076. Epub 2017 Dec 29. Carbohydr Polym. 2018. PMID: 29352919
-
Keratan sulfate (KS)-proteoglycans and neuronal regulation in health and disease: the importance of KS-glycodynamics and interactive capability with neuroregulatory ligands.J Neurochem. 2019 Apr;149(2):170-194. doi: 10.1111/jnc.14652. Epub 2019 Jan 27. J Neurochem. 2019. PMID: 30578672 Review.
-
Sulfated glycosaminoglycans: their distinct roles in stem cell biology.Glycoconj J. 2017 Dec;34(6):725-735. doi: 10.1007/s10719-016-9732-9. Epub 2016 Oct 6. Glycoconj J. 2017. PMID: 27709407 Review.
Cited by
-
(Semi)-Synthetic Fucosylated Chondroitin Sulfate Oligo- and Polysaccharides.Mar Drugs. 2020 Jun 1;18(6):293. doi: 10.3390/md18060293. Mar Drugs. 2020. PMID: 32492857 Free PMC article. Review.
-
Isolation of Keratan Sulfate Disaccharide-branched Chondroitin Sulfate E from Mactra chinensis.Bio Protoc. 2017 Aug 5;7(15):e2441. doi: 10.21769/BioProtoc.2441. eCollection 2017 Aug 5. Bio Protoc. 2017. PMID: 34541160 Free PMC article.
-
Branched Chondroitin Sulfate Oligosaccharides Derived from the Sea Cucumber Acaudina molpadioides Stimulate Neurite Outgrowth.Mar Drugs. 2022 Oct 21;20(10):653. doi: 10.3390/md20100653. Mar Drugs. 2022. PMID: 36286476 Free PMC article.
-
Cloning and Expression of Recombinant Chondroitinase ACII and Its Comparison to the Arthrobacter aurescens Enzyme.Biotechnol J. 2017 Oct;12(10):10.1002/biot.201700239. doi: 10.1002/biot.201700239. Epub 2017 Sep 4. Biotechnol J. 2017. PMID: 28799715 Free PMC article.
-
Galactosaminoglycans: Medical Applications and Drawbacks.Molecules. 2019 Aug 1;24(15):2803. doi: 10.3390/molecules24152803. Molecules. 2019. PMID: 31374852 Free PMC article. Review.
References
-
- Roden L. (1980) Structure and Metabolism of Connective Tissue Proteoglycans, Plenum Press, New York
-
- Volpi N.E. (2006) Chondroitin Sulfate: Structure, Role and Pharmacological Activity, Elsevier, London, UK
-
- Higashi K., Okamoto Y., Mano T., Wada T. and Toida T. (2014) A simple HPLC method for identification of the origin of chondroitin sulfate in health food. Jpn. J. Food Chem. Saf. 21, 187–194
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

