Brain Responses during the Anticipation of Dyspnea
- PMID: 27648309
- PMCID: PMC5018326
- DOI: 10.1155/2016/6434987
Brain Responses during the Anticipation of Dyspnea
Abstract
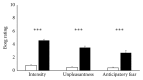
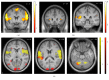
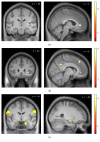
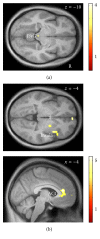
Dyspnea is common in many cardiorespiratory diseases. Already the anticipation of this aversive symptom elicits fear in many patients resulting in unfavorable health behaviors such as activity avoidance and sedentary lifestyle. This study investigated brain mechanisms underlying these anticipatory processes. We induced dyspnea using resistive-load breathing in healthy subjects during functional magnetic resonance imaging. Blocks of severe and mild dyspnea alternated, each preceded by anticipation periods. Severe dyspnea activated a network of sensorimotor, cerebellar, and limbic areas. The left insular, parietal opercular, and cerebellar cortices showed increased activation already during dyspnea anticipation. Left insular and parietal opercular cortex showed increased connectivity with right insular and anterior cingulate cortex when severe dyspnea was anticipated, while the cerebellum showed increased connectivity with the amygdala. Notably, insular activation during dyspnea perception was positively correlated with midbrain activation during anticipation. Moreover, anticipatory fear was positively correlated with anticipatory activation in right insular and anterior cingulate cortex. The results demonstrate that dyspnea anticipation activates brain areas involved in dyspnea perception. The involvement of emotion-related areas such as insula, anterior cingulate cortex, and amygdala during dyspnea anticipation most likely reflects anticipatory fear and might underlie the development of unfavorable health behaviors in patients suffering from dyspnea.
Figures

References
-
- Parshall M. B., Schwartzstein R. M., Adams L., et al. An official American thoracic society statement: update on the mechanisms, assessment, and management of dyspnea. American Journal of Respiratory and Critical Care Medicine. 2012;185(4):435–452. doi: 10.1164/rccm.201111-2042st. - DOI - PMC - PubMed
-
- Spruit M. A., Singh S. J., Garvey C., et al. An official American Thoracic Society/European Respiratory Society statement: key concepts and advances in pulmonary rehabilitation. American Journal of Respiratory and Critical Care Medicine. 2013;188(8):e13–e64. doi: 10.1164/rccm.201309-1634st. - DOI - PubMed
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

