RGDfK-functionalized gold nanorods bind only to activated platelets
- PMID: 27648522
- PMCID: PMC5657486
- DOI: 10.1002/jbm.a.35902
RGDfK-functionalized gold nanorods bind only to activated platelets
Abstract
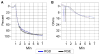
Integrin-targeting peptide RGDfK-labeled gold nanorods (GNR) seek to improve hyperthermia targeted to solid tumors by exploiting the known up-regulation of integrin αvβ3 cell membrane proteins on solid tumor vasculature surfaces. Tumor binding specificity might be expected since surrounding tissues and endothelial cells have limited numbers of these receptors. However, RGD peptide binding to many proteins is promiscuous, with known affinity to several families of cell integrin receptors, and also possible binding to platelets after intravenous infusion via a different integrin receptor, αIIbβ3, on platelets. Binding of RGDfK-targeted GNR could considerably impact platelet function, ultimately leading to increased risk of bleeding or thrombosis depending on the degree of interaction. We sought to determine if RGDfK-labeled GNR could interact with platelets and alter platelet function. Targeted and untargeted nanorods exhibited little interaction with resting platelets in platelet rich plasma (PRP) preparations. However, upon platelet activation, peptide-targeted nanorods bound actively to platelets. Addition of RGDfK-GNR to unactivated platelets had little effect on markers of platelet activation, indicating that RGDfK-nanorods were incapable of inducing platelet activation. We next tested whether activated platelet function was altered in the presence of peptide-targeted nanorods. Platelet aggregation in whole blood and PRP in the presence of targeted nanorods had no significant effect on platelet aggregation. These data suggest that RGDfK-GNR alone have little impact on platelet function in plasma. However, nonspecific nanorod binding may occur in vascular beds where activated platelets are normally cleared, such as the spleen and liver, producing a possible toxicity risk for these nanomaterials. © 2016 Wiley Periodicals, Inc. J Biomed Mater Res Part A: 105A: 209-217, 2017.
Keywords: activation; blood clot; coagulation; integrin; nanoparticles; peptide ligand.
© 2016 Wiley Periodicals, Inc.
Figures


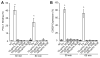
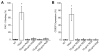
References
-
- Mizejewski GJ. Role of integrins in cancer: Survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124–138. - PubMed
-
- Garanger E, Boturyn D, Dumy P. Tumor targeting with RGD peptide ligands-design of new molecular conjugates for imaging and therapy of cancers. Anticancer Agents Med Chem. 2007;7:552–558. - PubMed
-
- Schottelius M, Laufer B, Kessler H, Wester HJ. Ligands for mapping alphavbeta3-integrin expression in vivo. Acc Chem Res. 2009;42:969–980. - PubMed
-
- Auzzas L, Zanardi F, Battistini L, Burreddu P, Carta P, Rassu G, Curti C, Casiraghi G. Targeting alphavbeta3 integrin: Design and applications of mono- and multifunctional RGD-based peptides and semipeptides. Curr Med Chem. 2010;17:1255–1299. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

