Control of Meiotic Crossovers: From Double-Strand Break Formation to Designation
- PMID: 27648641
- PMCID: PMC5319444
- DOI: 10.1146/annurev-genet-120215-035111
Control of Meiotic Crossovers: From Double-Strand Break Formation to Designation
Abstract
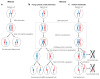
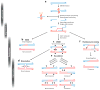
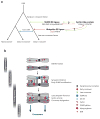
Meiosis, the mechanism of creating haploid gametes, is a complex cellular process observed across sexually reproducing organisms. Fundamental to meiosis is the process of homologous recombination, whereby DNA double-strand breaks are introduced into the genome and are subsequently repaired to generate either noncrossovers or crossovers. Although homologous recombination is essential for chromosome pairing during prophase I, the resulting crossovers are critical for maintaining homolog interactions and enabling accurate segregation at the first meiotic division. Thus, the placement, timing, and frequency of crossover formation must be exquisitely controlled. In this review, we discuss the proteins involved in crossover formation, the process of their formation and designation, and the rules governing crossovers, all within the context of the important landmarks of prophase I. We draw together crossover designation data across organisms, analyze their evolutionary divergence, and propose a universal model for crossover regulation.
Keywords: crossover designation; homologous recombination; meiosis.
Figures


References
-
- Agarwal S, Roeder GS. Zip3 provides a link between recombination enzymes and synaptonemal complex proteins. Cell. 2000;102(2):245–55. - PubMed
-
- Allers T, Lichten M. Intermediates of yeast meiotic recombination contain heteroduplex DNA. Mol Cell. 2001;8(1):225–31. - PubMed
-
- Allers T, Lichten M. Differential timing and control of noncrossover and crossover recombination during meiosis. Cell. 2001;106(1):47–57. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

