HIV-1 Vpr Abrogates the Effect of TSG101 Overexpression to Support Virus Release
- PMID: 27648839
- PMCID: PMC5029901
- DOI: 10.1371/journal.pone.0163100
HIV-1 Vpr Abrogates the Effect of TSG101 Overexpression to Support Virus Release
Abstract
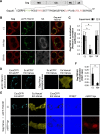
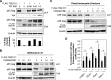
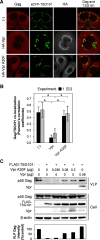
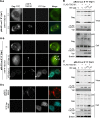
HIV-1 budding requires interaction between Gag and cellular TSG101 to initiate viral particle assembly and release via the endosomal sorting complexes required for transport (ESCRT) pathway. However, some reports show that overexpression of TSG101 inhibits virus release by disruption of Gag targeting process. Since a HIV-1 accessory protein, Vpr binds to Gag p6 domain at the position close to the binding site for TSG101, whether Vpr implicates TSG101 overexpression effect has not been investigated. Here, we found that Vpr abrogates TSG101 overexpression effect to rescue viral production. Co-transfection of TSG101 and Gag with Vpr prevented TSG101-induced Gag accumulation in endosomes and lysosomes. In addition, Vpr rescued virus-like particle (VLP) production in a similar manner as a lysosomal inhibitor, Bafilomycin A1 indicating that Vpr inhibits TSG101-induced Gag downregulation via lysosomal pathway. Vpr and Gag interaction is required to counteract TSG101 overexpression effect since Vpr A30F mutant which is unable to interact with Gag and incorporate into virions, reduced ability to prevent Gag accumulation and to rescue VLP production. In addition, GST pull-down assays and Biacore analysis revealed that Vpr competed with TSG101 for Gag binding. These results indicate that Vpr overcomes the effects of TSG101 overexpression to support viral production by competing with TSG101 to bind Gag.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures
Similar articles
-
Defects in human immunodeficiency virus budding and endosomal sorting induced by TSG101 overexpression.J Virol. 2003 Jun;77(11):6507-19. doi: 10.1128/jvi.77.11.6507-6519.2003. J Virol. 2003. PMID: 12743307 Free PMC article.
-
The C-terminal portion of the Hrs protein interacts with Tsg101 and interferes with human immunodeficiency virus type 1 Gag particle production.J Virol. 2007 Mar;81(6):2909-22. doi: 10.1128/JVI.01413-06. Epub 2006 Dec 20. J Virol. 2007. PMID: 17182674 Free PMC article.
-
Tsg101 control of human immunodeficiency virus type 1 Gag trafficking and release.J Virol. 2003 Sep;77(17):9173-82. doi: 10.1128/jvi.77.17.9173-9182.2003. J Virol. 2003. PMID: 12915533 Free PMC article.
-
Novel Tsg101 Binding Partners Regulate Viral L Domain Trafficking.Viruses. 2021 Jun 15;13(6):1147. doi: 10.3390/v13061147. Viruses. 2021. PMID: 34203832 Free PMC article. Review.
-
[The action of TSG101 on HIV-1 budding and related inhibitors].Yao Xue Xue Bao. 2008 Dec;43(12):1165-70. Yao Xue Xue Bao. 2008. PMID: 19244744 Review. Chinese.
Cited by
-
Huntingtin-Interacting Protein 1 Promotes Vpr-Induced G2 Arrest and HIV-1 Infection in Macrophages.Viruses. 2021 Nov 19;13(11):2308. doi: 10.3390/v13112308. Viruses. 2021. PMID: 34835114 Free PMC article.
-
HIV-1 Vpr Functions in Primary CD4+ T Cells.Viruses. 2024 Mar 9;16(3):420. doi: 10.3390/v16030420. Viruses. 2024. PMID: 38543785 Free PMC article. Review.
References
-
- Garrus JE, von Schwedler UK, Pornillos OW, Morham SG, Zavitz KH, Wang HE, et al. Tsg101 and the vacuolar protein sorting pathway are essential for HIV-1 budding. Cell. 2001;107(1):55–65. Epub 2001/10/12. . - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

