Hydrogen Bonding and Dielectric Spectra of Ethylene Glycol-Water Mixtures from Molecular Dynamics Simulations
- PMID: 27649083
- PMCID: PMC5066130
- DOI: 10.1021/acs.jpcb.6b05236
Hydrogen Bonding and Dielectric Spectra of Ethylene Glycol-Water Mixtures from Molecular Dynamics Simulations
Abstract
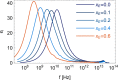
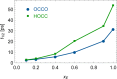
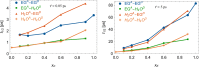
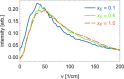
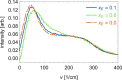
Mixtures of ethylene glycol with water are a prominent example of media with variable viscosity. Classical molecular dynamics simulations at room temperature were performed for mixtures of ethylene glycol (EG) and water with EG mole fractions of xE = 0.0, 0.1, 0.2, 0.4, 0.6, 0.9, 1.0. The calculated dielectric loss spectra were in qualitative agreement with experiment. We found a slightly overestimated slowdown of the dynamics with increasing EG concentration compared to experimental data. Statistics of the hydrogen bond network and hydrogen bond lifetimes were derived from suitable time correlation functions and also show a slowdown in the dynamics with increasing xE. A similar picture is predicted for the time scales of EG conformer changes and for molecular reorientation. A slight blue shift was obtained for the power spectra of the molecular center of mass motion. The results were used to give a qualitative interpretation of the origin of three different relaxation times that appear in experimental complex dielectric spectra and of their change with xE.
Conflict of interest statement
The authors declare no competing financial interest.
Figures


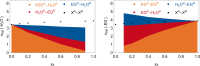
References
-
- Zhang J.; Zhang P.; Ma K.; Han F.; Chen G.; Wei X. Hydrogen bonding interactions between ethylene glycol and water: density, excess molar volume, and spectral study. Sci. China, Ser. B: Chem. 2008, 51, 420–426. 10.1007/s11426-008-0045-0. - DOI
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

