ER Stress-Mediated Signaling: Action Potential and Ca(2+) as Key Players
- PMID: 27649160
- PMCID: PMC5037829
- DOI: 10.3390/ijms17091558
ER Stress-Mediated Signaling: Action Potential and Ca(2+) as Key Players
Abstract
The proper functioning of the endoplasmic reticulum (ER) is crucial for multiple cellular activities and survival. Disturbances in the normal ER functions lead to the accumulation and aggregation of unfolded proteins, which initiates an adaptive response, the unfolded protein response (UPR), in order to regain normal ER functions. Failure to activate the adaptive response initiates the process of programmed cell death or apoptosis. Apoptosis plays an important role in cell elimination, which is essential for embryogenesis, development, and tissue homeostasis. Impaired apoptosis can lead to the development of various pathological conditions, such as neurodegenerative and autoimmune diseases, cancer, or acquired immune deficiency syndrome (AIDS). Calcium (Ca(2+)) is one of the key regulators of cell survival and it can induce ER stress-mediated apoptosis in response to various conditions. Ca(2+) regulates cell death both at the early and late stages of apoptosis. Severe Ca(2+) dysregulation can promote cell death through apoptosis. Action potential, an electrical signal transmitted along the neurons and muscle fibers, is important for conveying information to, from, and within the brain. Upon the initiation of the action potential, increased levels of cytosolic Ca(2+) (depolarization) lead to the activation of the ER stress response involved in the initiation of apoptosis. In this review, we discuss the involvement of Ca(2+) and action potential in ER stress-mediated apoptosis.
Keywords: action potential; apoptosis; calcium; endoplasmic reticulum stress; unfolded protein response.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
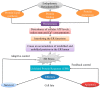
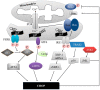
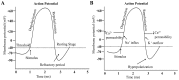
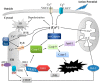
References
-
- Benyair R., Ron E., Lederkremer G.Z. Protein quality control, retention, and degradation at the endoplasmic reticulum. Int. Rev. Cell Mol. Biol. 2011;292:197–280. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

