Transmembrane Substrate Determinants for γ-Secretase Processing of APP CTFβ
- PMID: 27649271
- PMCID: PMC5539764
- DOI: 10.1021/acs.biochem.6b00718
Transmembrane Substrate Determinants for γ-Secretase Processing of APP CTFβ
Abstract
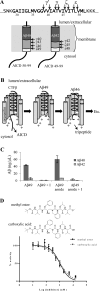
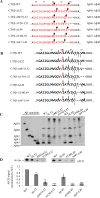
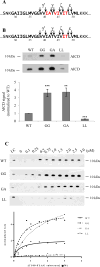
The amyloid β-peptide (Aβ) of Alzheimer's disease (AD) is generated by proteolysis within the transmembrane domain (TMD) of a C-terminal fragment of the amyloid β protein-precursor (APP CTFβ) by the γ-secretase complex. This processing produces Aβ ranging from 38 to 49 residues in length. Evidence suggests that this spectrum of Aβ peptides is the result of successive γ-secretase cleavages, with endoproteolysis first occurring at the ε sites to generate Aβ48 or Aβ49, followed by C-terminal trimming mostly every three residues along two product lines to generate shorter, secreted forms of Aβ: the primary Aβ49-46-43-40 line and a minor Aβ48-45-42-38 line. The major secreted Aβ species are Aβ40 and Aβ42, and an increased proportion of the longer, aggregation-prone Aβ42 compared to Aβ40 is widely thought to be important in AD pathogenesis. We examined TMD substrate determinants of the specificity and efficiency of ε site endoproteolysis and carboxypeptidase trimming of CTFβ by γ-secretase. We determined that the C-terminal negative charge of the intermediate Aβ49 does not play a role in its trimming by γ-secretase. Peptidomimetic probes suggest that γ-secretase has S1', S2', and S3' pockets, through which trimming by tripeptides may be determined. However, deletion of residues around the ε sites demonstrates that a depth of three residues within the TMD is not a determinant of the location of endoproteolytic ε cleavage of CTFβ. We also show that instability of the CTFβ TMD helix near the ε site significantly increases endoproteolysis, and that helical instability near the carboxypeptidase cleavage sites facilitates C-terminal trimming by γ-secretase. In addition, we found that CTFβ dimers are not endoproteolyzed by γ-secretase. These results support a model in which initial interaction of the array of residues along the undimerized single helical TMD of substrates dictates the site of initial ε cleavage and that helix unwinding is essential for both endoproteolysis and carboxypeptidase trimming.
Figures




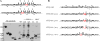
References
-
- Jarrett JT, Berger EP, Lansbury PT., Jr The carboxy terminus of the beta amyloid protein is critical for the seeding of amyloid formation: implications for the pathogenesis of Alzheimer’s disease. Biochemistry. 1993;32:4693–4697. - PubMed
-
- Iwatsubo T, Odaka A, Suzuki N, Mizusawa H, Nukina N, Ihara Y. Visualization of A beta 42(43) and A beta 40 in senile plaques with end-specific A beta monoclonals: evidence that an initially deposited species is A beta 42(43) Neuron. 1994;13:45–53. - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

