BMP Signaling Mediated by BMPR1A in Osteoclasts Negatively Regulates Osteoblast Mineralization Through Suppression of Cx43
- PMID: 27649478
- PMCID: PMC5813677
- DOI: 10.1002/jcb.25746
BMP Signaling Mediated by BMPR1A in Osteoclasts Negatively Regulates Osteoblast Mineralization Through Suppression of Cx43
Abstract
Osteoblasts and osteoclasts are well orchestrated through different mechanisms of communication during bone remodeling. Previously, we found that osteoclast-specific disruption of one of the BMP receptors, Bmpr1a, results in increased osteoblastic bone formation in mice. We hypothesized that BMPR1A signaling in osteoclasts regulates production of either membrane bound proteins or secreted molecules that regulated osteoblast differentiation. In our current study, we co-cultured wild-type osteoblasts with either control osteoclasts or osteoclasts lacking BMPR1A signaling activity. We found that loss of Bmpr1a in osteoclasts promoted osteoblast mineralization in vitro. Further, we found that the expression of Cx43/Gja1 in the mutant osteoclasts was increased, which encoded for one of the gap junction proteins connexin 43/gap junction alpha 1. Knockdown of Gja1 in the mutant osteoclasts for Bmpr1a reduced osteoblastic mineralization when co-cultured. Our findings suggest that GJA1 may be one of the downstream targets of BMPR1A signaling in osteoclasts that mediates osteoclast-osteoblast communication during bone remodeling. J. Cell. Biochem. 118: 605-614, 2017. © 2016 Wiley Periodicals, Inc.
Keywords: BMPR1A; CONNEXIN 43/GJA1; GAP JUNCTION; OSTEOBLAST; OSTEOCLAST.
© 2016 Wiley Periodicals, Inc.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article.
Figures

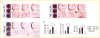
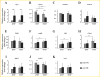


Similar articles
-
Conditional deletion of Bmpr1a in differentiated osteoclasts increases osteoblastic bone formation, increasing volume of remodeling bone in mice.J Bone Miner Res. 2011 Oct;26(10):2511-22. doi: 10.1002/jbmr.477. J Bone Miner Res. 2011. PMID: 21786321
-
Hyperthyroidism-driven bone loss depends on BMP receptor Bmpr1a expression in osteoblasts.Commun Biol. 2024 May 8;7(1):548. doi: 10.1038/s42003-024-06227-0. Commun Biol. 2024. PMID: 38719881 Free PMC article.
-
Connexin43 and Runx2 Interact to Affect Cortical Bone Geometry, Skeletal Development, and Osteoblast and Osteoclast Function.J Bone Miner Res. 2017 Aug;32(8):1727-1738. doi: 10.1002/jbmr.3152. Epub 2017 May 22. J Bone Miner Res. 2017. PMID: 28419546 Free PMC article.
-
The Role Of BMPs in the Regulation of Osteoclasts Resorption and Bone Remodeling: From Experimental Models to Clinical Applications.Front Immunol. 2022 Apr 26;13:869422. doi: 10.3389/fimmu.2022.869422. eCollection 2022. Front Immunol. 2022. PMID: 35558080 Free PMC article. Review.
-
Molecular mechanisms of osteoblast/osteocyte regulation by connexin43.Calcif Tissue Int. 2014 Jan;94(1):55-67. doi: 10.1007/s00223-013-9742-6. Epub 2013 Jun 11. Calcif Tissue Int. 2014. PMID: 23754488 Free PMC article. Review.
Cited by
-
Activin A receptor type 1-mediated BMP signaling regulates RANKL-induced osteoclastogenesis via canonical SMAD-signaling pathway.J Biol Chem. 2019 Nov 22;294(47):17818-17836. doi: 10.1074/jbc.RA119.009521. Epub 2019 Oct 16. J Biol Chem. 2019. PMID: 31619522 Free PMC article.
-
Vascular Calcification: New Insights Into BMP Type I Receptor A.Front Pharmacol. 2022 Apr 6;13:887253. doi: 10.3389/fphar.2022.887253. eCollection 2022. Front Pharmacol. 2022. PMID: 35462911 Free PMC article. Review.
-
[Latest Research Findings on the Regulation of Bone Homeostasis by ALK3].Sichuan Da Xue Xue Bao Yi Xue Ban. 2022 May;53(3):517-522. doi: 10.12182/20220560304. Sichuan Da Xue Xue Bao Yi Xue Ban. 2022. PMID: 35642164 Free PMC article. Review. Chinese.
-
Loss of Myeloid BMPR1a Alters Differentiation and Reduces Mouse Prostate Cancer Growth.Front Oncol. 2020 Apr 7;10:357. doi: 10.3389/fonc.2020.00357. eCollection 2020. Front Oncol. 2020. PMID: 32318332 Free PMC article.
-
A Synthetic Peptide, CK2.3, Inhibits RANKL-Induced Osteoclastogenesis through BMPRIa and ERK Signaling Pathway.J Dev Biol. 2020 Jul 9;8(3):12. doi: 10.3390/jdb8030012. J Dev Biol. 2020. PMID: 32660129 Free PMC article.
References
-
- Atanga E, Dolder S, Dauwalder T, Wetterwald A, Hofstetter W. TNFalpha inhibits the development of osteoclasts through osteoblast-derived GM-CSF. Bone. 2011;49:1090–1100. - PubMed
-
- Bani-Yaghoub M, Felker JM, Sans C, Naus CC. The effects of bone morphogenetic protein 2 and 4 (BMP2 and BMP4) on gap junctions during neurodevelopment. Exp Neurol. 2000;162:13–26. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

