Upper Limb Evaluation in Duchenne Muscular Dystrophy: Fat-Water Quantification by MRI, Muscle Force and Function Define Endpoints for Clinical Trials
- PMID: 27649492
- PMCID: PMC5029878
- DOI: 10.1371/journal.pone.0162542
Upper Limb Evaluation in Duchenne Muscular Dystrophy: Fat-Water Quantification by MRI, Muscle Force and Function Define Endpoints for Clinical Trials
Abstract
Objective: A number of promising experimental therapies for Duchenne muscular dystrophy (DMD) are emerging. Clinical trials currently rely on invasive biopsies or motivation-dependent functional tests to assess outcome. Quantitative muscle magnetic resonance imaging (MRI) could offer a valuable alternative and permit inclusion of non-ambulant DMD subjects. The aims of our study were to explore the responsiveness of upper-limb MRI muscle-fat measurement as a non-invasive objective endpoint for clinical trials in non-ambulant DMD, and to investigate the relationship of these MRI measures to those of muscle force and function.
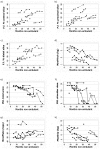
Methods: 15 non-ambulant DMD boys (mean age 13.3 y) and 10 age-gender matched healthy controls (mean age 14.6 y) were recruited. 3-Tesla MRI fat-water quantification was used to measure forearm muscle fat transformation in non-ambulant DMD boys compared with healthy controls. DMD boys were assessed at 4 time-points over 12 months, using 3-point Dixon MRI to measure muscle fat-fraction (f.f.). Images from ten forearm muscles were segmented and mean f.f. and cross-sectional area recorded. DMD subjects also underwent comprehensive upper limb function and force evaluation.
Results: Overall mean baseline forearm f.f. was higher in DMD than in healthy controls (p<0.001). A progressive f.f. increase was observed in DMD over 12 months, reaching significance from 6 months (p<0.001, n = 7), accompanied by a significant loss in pinch strength at 6 months (p<0.001, n = 9) and a loss of upper limb function and grip force observed over 12 months (p<0.001, n = 8).
Conclusions: These results support the use of MRI muscle f.f. as a biomarker to monitor disease progression in the upper limb in non-ambulant DMD, with sensitivity adequate to detect group-level change over time intervals practical for use in clinical trials. Clinical validity is supported by the association of the progressive fat transformation of muscle with loss of muscle force and function.
Conflict of interest statement
RLJ is an employee of GlaxoSmithKline. PMM was an employee of GlaxoSmithKline during design and initial implementation stages of this study. FM also received funding from GlaxoSmithKline. TAY has received honoraria and travel expenses for advisory committee work from Bayer Schering, Biogen Idec, and Novartis; and research grants (held by University College London) from Biogen Idec, GlaxoSmithKline, Novartis, and Schering AG for analysis of data from MS trials. MMR received grant funding from the National Institute of Neurological Disorders and Stroke/Office of Rare Diseases and the Medical Research Council (MRC). MGH is supported by the MRC Centre and by The Myositis Support Group. FM has served on scientific advisory boards for Acceleron Pharma, Genzyme,AVI BioPharma, Debiopharma Group, GlaxoSmithKline, Prosensa, Servier, Summit and Santhera Pharmaceutical, receives research support from Trophos, and GlaxoSmithKline, and has received funding for trials from AVI, and PTC. JST has received research support from GlaxoSmithKline, Medtronic and Siemens. JYH is an inventor of the MyoGrip (patent pending, PCT/FR2013/050694), MyoPinch (patent pending, PCT/2013/052106) and MoviPlate (patent pending, PCT/2013/050353) devices. VR is currently an employee of Biomarin Europe. This does not alter our adherence to PLOS ONE policies on sharing data and materials. The other authors have no conflict of interest to declare.
Figures


References
-
- Ricotti V, Ridout DA, Scott E, Quinlivan R, Robb SA, Manzur AY, et al. Long-term benefits and adverse effects of intermittent versus daily glucocorticoids in boys with Duchenne muscular dystrophy. J Neurol Neurosurg Psychiatry. 2013;84(6):698–705. Epub 2012/12/20. 10.1136/jnnp-2012-303902 . - DOI - PubMed
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

