High Throughput Random Mutagenesis and Single Molecule Real Time Sequencing of the Muscle Nicotinic Acetylcholine Receptor
- PMID: 27649498
- PMCID: PMC5029940
- DOI: 10.1371/journal.pone.0163129
High Throughput Random Mutagenesis and Single Molecule Real Time Sequencing of the Muscle Nicotinic Acetylcholine Receptor
Abstract
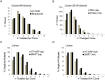
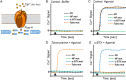
High throughput random mutagenesis is a powerful tool to identify which residues are important for the function of a protein, and gain insight into its structure-function relation. The human muscle nicotinic acetylcholine receptor was used to test whether this technique previously used for monomeric receptors can be applied to a pentameric ligand-gated ion channel. A mutant library for the α1 subunit of the channel was generated by error-prone PCR, and full length sequences of all 2816 mutants were retrieved using single molecule real time sequencing. Each α1 mutant was co-transfected with wildtype β1, δ, and ε subunits, and the channel function characterized by an ion flux assay. To test whether the strategy could map the structure-function relation of this receptor, we attempted to identify mutations that conferred resistance to competitive antagonists. Mutant hits were defined as receptors that responded to the nicotinic agonist epibatidine, but were not inhibited by either α-bungarotoxin or tubocurarine. Eight α1 subunit mutant hits were identified, six of which contained mutations at position Y233 or V275 in the transmembrane domain. Three single point mutations (Y233N, Y233H, and V275M) were studied further, and found to enhance the potencies of five channel agonists tested. This suggests that the mutations made the channel resistant to the antagonists, not by impairing antagonist binding, but rather by producing a gain-of-function phenotype, e.g. increased agonist sensitivity. Our data show that random high throughput mutagenesis is applicable to multimeric proteins to discover novel functional mutants, and outlines the benefits of using single molecule real time sequencing with regards to quality control of the mutant library as well as downstream mutant data interpretation.
Conflict of interest statement
All authors except L.G. Sivilotti are employed by the Novartis Institutes for BioMedical Research. This does not alter our adherence to PLOS ONE policies on sharing data and materials.
Figures



Similar articles
-
Long-term exposure to the new nicotinic antagonist 1,2-bisN-cytisinylethane upregulates nicotinic receptor subtypes of SH-SY5Y human neuroblastoma cells.Br J Pharmacol. 2005 Dec;146(8):1096-109. doi: 10.1038/sj.bjp.0706434. Br J Pharmacol. 2005. PMID: 16273122 Free PMC article.
-
Distinctions in agonist and antagonist specificity conferred by anionic residues of the nicotinic acetylcholine receptor.J Biol Chem. 1998 May 22;273(21):12758-65. doi: 10.1074/jbc.273.21.12758. J Biol Chem. 1998. PMID: 9582301
-
Topology of ligand binding sites on the nicotinic acetylcholine receptor.Brain Res Brain Res Rev. 1997 Oct;25(2):133-91. doi: 10.1016/s0165-0173(97)00020-9. Brain Res Brain Res Rev. 1997. PMID: 9403137 Review.
-
Roles of amino acids and subunits in determining the inhibition of nicotinic acetylcholine receptors by competitive antagonists.Anesthesiology. 2007 Jun;106(6):1186-95. doi: 10.1097/01.anes.0000267602.94516.7f. Anesthesiology. 2007. PMID: 17525594 Free PMC article.
-
The emerging three-dimensional structure of a receptor. The nicotinic acetylcholine receptor.Eur J Biochem. 1996 Aug 1;239(3):539-57. doi: 10.1111/j.1432-1033.1996.0539u.x. Eur J Biochem. 1996. PMID: 8774696 Review.
Cited by
-
Deep mutational scanning of proteins in mammalian cells.Cell Rep Methods. 2023 Nov 20;3(11):100641. doi: 10.1016/j.crmeth.2023.100641. Epub 2023 Nov 13. Cell Rep Methods. 2023. PMID: 37963462 Free PMC article. Review.
-
Curare alkaloids from Matis Dart Poison: Comparison with d-tubocurarine in interactions with nicotinic, 5-HT3 serotonin and GABAA receptors.PLoS One. 2019 Jan 4;14(1):e0210182. doi: 10.1371/journal.pone.0210182. eCollection 2019. PLoS One. 2019. PMID: 30608952 Free PMC article.
-
OverFlap PCR: A reliable approach for generating plasmid DNA libraries containing random sequences without a template bias.PLoS One. 2022 Aug 8;17(8):e0262968. doi: 10.1371/journal.pone.0262968. eCollection 2022. PLoS One. 2022. PMID: 35939421 Free PMC article.
-
Development of a species-specific transformation system using the novel endogenous promoter calreticulin from oleaginous microalgae Ettlia sp.Sci Rep. 2020 Aug 18;10(1):13947. doi: 10.1038/s41598-020-70503-2. Sci Rep. 2020. PMID: 32811857 Free PMC article.
References
-
- Unwin N. Refined structure of the nicotinic acetylcholine receptor at 4A resolution. J Mol Biol. 2005; 346: 967–989. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources

