Relationship of clusterin with renal inflammation and fibrosis after the recovery phase of ischemia-reperfusion injury
- PMID: 27649757
- PMCID: PMC5028988
- DOI: 10.1186/s12882-016-0348-x
Relationship of clusterin with renal inflammation and fibrosis after the recovery phase of ischemia-reperfusion injury
Abstract
Background: Long-term outcomes after acute kidney injury (AKI) include incremental loss of function and progression towards chronic kidney disease (CKD); however, the pathogenesis of AKI to CKD remains largely unknown. Clusterin (CLU) is a chaperone-like protein that reduces ischemia-reperfusion injury (IRI) and enhances tissue repair after IRI in the kidney. This study investigated the role of CLU in the transition of IRI to renal fibrosis.
Methods: IRI was induced in the left kidneys of wild type (WT) C57BL/6J (B6) versus CLU knockout (KO) B6 mice by clamping the renal pedicles for 28 min at the body temperature of 32 °C. Tissue damage was examined by histology, infiltrate phenotypes by flow cytometry analysis, and fibrosis-related gene expression by PCR array.
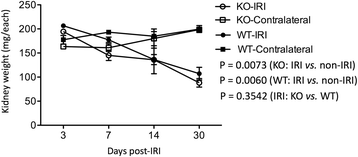
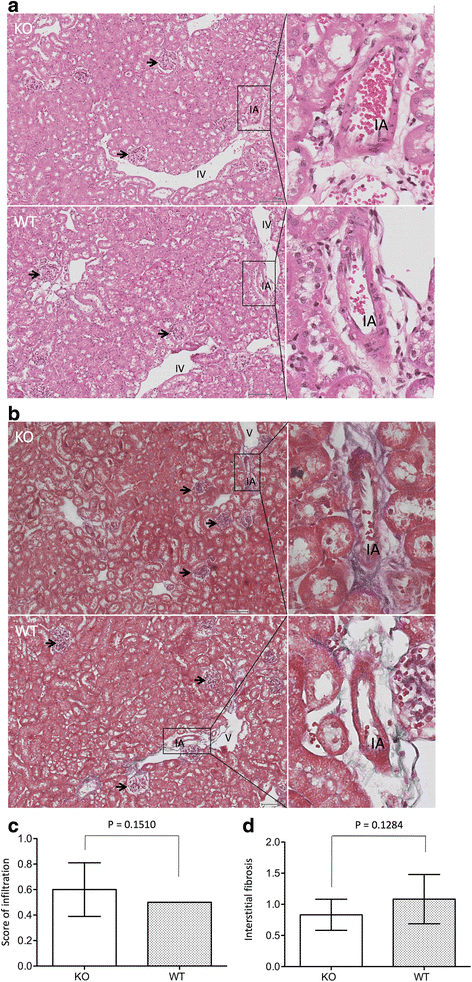
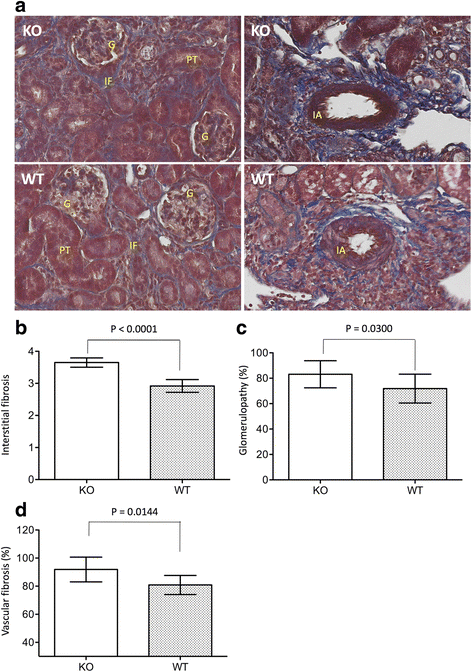
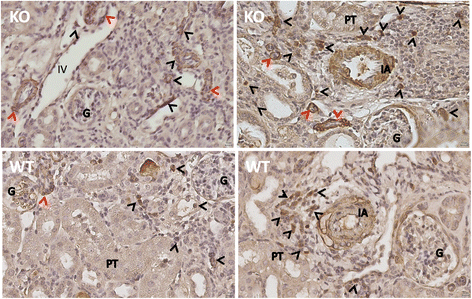
Results: Reduction of kidney weight was induced by IRI, but was not affected by CLU KO. Both WT and KO kidneys had similar function with minimal cellular infiltration and fibrosis at day 14 of reperfusion. After 30 days, KO kidneys had greater loss in function than WT, indicated by the higher levels of both serum creatinine and BUN in KO mice, and exhibited more cellular infiltration (CD8 cells and macrophages), more tubular damage and more severe tissue fibrosis (glomerulopathy, interstitial fibrosis and vascular fibrosis). PCR array showed the association of CLU deficiency with up-regulation of CCL12, Col3a1, MMP9 and TIMP1 and down-regulation of EGF in these kidneys.
Conclusion: Our data suggest that CLU deficiency worsens renal inflammation and tissue fibrosis after IRI in the kidney, which may be mediated through multiple pathways.
Keywords: Acute kidney injury; Chronic kidney disease; Clusterin; Fibrosis; Kidney ischemia-reperfusion.
Figures




Similar articles
-
Promotion of cell proliferation by clusterin in the renal tissue repair phase after ischemia-reperfusion injury.Am J Physiol Renal Physiol. 2014 Apr 1;306(7):F724-33. doi: 10.1152/ajprenal.00410.2013. Epub 2014 Jan 29. Am J Physiol Renal Physiol. 2014. PMID: 24477687
-
Loss of clusterin expression worsens renal ischemia-reperfusion injury.Am J Physiol Renal Physiol. 2010 Mar;298(3):F568-78. doi: 10.1152/ajprenal.00399.2009. Epub 2009 Dec 9. Am J Physiol Renal Physiol. 2010. PMID: 20007348
-
Clusterin regulates macrophage expansion, polarization and phagocytic activity in response to inflammation in the kidneys.Immunol Cell Biol. 2021 Mar;99(3):274-287. doi: 10.1111/imcb.12405. Epub 2020 Oct 16. Immunol Cell Biol. 2021. PMID: 32935392 Free PMC article.
-
Rodent models of AKI-CKD transition.Am J Physiol Renal Physiol. 2018 Oct 1;315(4):F1098-F1106. doi: 10.1152/ajprenal.00199.2018. Epub 2018 Jun 27. Am J Physiol Renal Physiol. 2018. PMID: 29949392 Free PMC article. Review.
-
Role of Complement Properdin in Renal Ischemia-Reperfusion Injury.Curr Gene Ther. 2017;17(6):411-423. doi: 10.2174/1566523218666180214093043. Curr Gene Ther. 2017. PMID: 29446739 Review.
Cited by
-
Advances in the study of subclinical AKI biomarkers.Front Physiol. 2022 Aug 24;13:960059. doi: 10.3389/fphys.2022.960059. eCollection 2022. Front Physiol. 2022. PMID: 36091391 Free PMC article. Review.
-
Glomerular clusterin expression is increased in diabetic nephropathy and protects against oxidative stress-induced apoptosis in podocytes.Sci Rep. 2020 Sep 10;10(1):14888. doi: 10.1038/s41598-020-71629-z. Sci Rep. 2020. PMID: 32913257 Free PMC article.
-
The Ins and Outs of Clusterin: Its Role in Cancer, Eye Diseases and Wound Healing.Int J Mol Sci. 2023 Aug 24;24(17):13182. doi: 10.3390/ijms241713182. Int J Mol Sci. 2023. PMID: 37685987 Free PMC article. Review.
-
Unveiling the role of transgelin as a prognostic and therapeutic target in kidney fibrosis via a proteomic approach.Exp Mol Med. 2024 Oct;56(10):2296-2308. doi: 10.1038/s12276-024-01319-7. Epub 2024 Oct 7. Exp Mol Med. 2024. PMID: 39375532 Free PMC article.
-
Exploring diagnostic biomarkers of type 2 cardio-renal syndrome based on secreted proteins and bioinformatics analysis.Sci Rep. 2024 Oct 19;14(1):24612. doi: 10.1038/s41598-024-75580-1. Sci Rep. 2024. PMID: 39427047 Free PMC article.
References
-
- Bahloul M, Ben Hamida C, Damak H, Kallel H, Ksibi H, Rekik N, et al. Incidence and prognosis of acute renal failure in the intensive care unit. Retrospective study of 216 cases. Tunis Med. 2003;81(4):250–7. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

