HbE/β-Thalassemia and Oxidative Stress: The Key to Pathophysiological Mechanisms and Novel Therapeutics
- PMID: 27650096
- PMCID: PMC5421591
- DOI: 10.1089/ars.2016.6806
HbE/β-Thalassemia and Oxidative Stress: The Key to Pathophysiological Mechanisms and Novel Therapeutics
Abstract
Significance: Oxidative stress and generation of free radicals are fundamental in initiating pathophysiological mechanisms leading to an inflammatory cascade resulting in high rates of morbidity and death from many inherited point mutation-derived hemoglobinopathies. Hemoglobin (Hb)E is the most common point mutation worldwide. The βE-globin gene is found in greatest frequency in Southeast Asia, including Thailand, Malaysia, Indonesia, Vietnam, Cambodia, and Laos. With the wave of worldwide migration, it is entering the gene pool of diverse populations with greater consequences than expected.
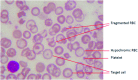
Critical issues: While HbE by itself presents as a mild anemia and a single gene for β-thalassemia is not serious, it remains unexplained why HbE/β-thalassemia (HbE/β-thal) is a grave disease with high morbidity and mortality. Patients often exhibit defective physical development, severe chronic anemia, and often die of cardiovascular disease and severe infections. Recent Advances: This article presents an overview of HbE/β-thal disease with an emphasis on new findings pointing to pathophysiological mechanisms derived from and initiated by the dysfunctional property of HbE as a reduced nitrite reductase concomitant with excess α-chains exacerbating unstable HbE, leading to a combination of nitric oxide imbalance, oxidative stress, and proinflammatory events.
Future directions: Additionally, we present new therapeutic strategies that are based on the emerging molecular-level understanding of the pathophysiology of this and other hemoglobinopathies. These strategies are designed to short-circuit the inflammatory cascade leading to devastating chronic morbidity and fatal consequences. Antioxid. Redox Signal. 26, 794-813.
Keywords: hemoglobin E; hypoxia; inflammation; nitric oxide; oxidative stress; β-thalassemia.
Conflict of interest statement
J. M.F. is on the Science Advisory Board of Nano Biomed, Inc. R.E.H., S.F., and N.S. have no disclosures to report.
Figures







Similar articles
-
Oxidative instability of hemoglobin E (β26 Glu→Lys) is increased in the presence of free α subunits and reversed by α-hemoglobin stabilizing protein (AHSP): Relevance to HbE/β-thalassemia.Redox Biol. 2016 Aug;8:363-74. doi: 10.1016/j.redox.2016.03.004. Epub 2016 Mar 10. Redox Biol. 2016. PMID: 26995402 Free PMC article.
-
Is hemoglobin instability important in the interaction between hemoglobin E and beta thalassemia?Blood. 1998 Sep 15;92(6):2141-6. Blood. 1998. PMID: 9731073
-
Alpha globin gene numbers: an important modifier of HbE/beta thalassemia.Hematology. 2009 Oct;14(5):297-300. doi: 10.1179/102453309X446126. Hematology. 2009. PMID: 19843387
-
The hemoglobin E syndromes.Ann N Y Acad Sci. 1998 Jun 30;850:334-43. doi: 10.1111/j.1749-6632.1998.tb10490.x. Ann N Y Acad Sci. 1998. PMID: 9668555 Review.
-
Treatment strategies for hemoglobin E beta-thalassemia.Blood Rev. 2012 Apr;26 Suppl 1:S28-30. doi: 10.1016/S0268-960X(12)70009-7. Blood Rev. 2012. PMID: 22631039 Review.
Cited by
-
Impact of met-haemoglobin and oxidative stress on endothelial function in patients with transfusion dependent β-thalassemia.Sci Rep. 2024 Oct 25;14(1):25328. doi: 10.1038/s41598-024-74930-3. Sci Rep. 2024. PMID: 39455629 Free PMC article.
-
Thalassemia and Moyamoya syndrome: unfurling an intriguing association.J Neurol. 2019 Nov;266(11):2838-2847. doi: 10.1007/s00415-019-09497-5. Epub 2019 Aug 17. J Neurol. 2019. PMID: 31422456 Review.
-
Measures of cellular oxidative damage following vitamin E supplementation in young patients with transfusion-dependent thalassemia: a double-blind randomized controlled trial.BMC Pediatr. 2025 May 20;25(1):405. doi: 10.1186/s12887-025-05741-2. BMC Pediatr. 2025. PMID: 40389864 Free PMC article. Clinical Trial.
-
Case report: A rare heterozygous Hb CS with heterozygous HbE in a family with thalassemia in China.Heliyon. 2024 Sep 12;10(18):e37858. doi: 10.1016/j.heliyon.2024.e37858. eCollection 2024 Sep 30. Heliyon. 2024. PMID: 39323799 Free PMC article.
-
Genetic correction of haemoglobin E in an immortalised haemoglobin E/beta-thalassaemia cell line using the CRISPR/Cas9 system.Sci Rep. 2022 Sep 16;12(1):15551. doi: 10.1038/s41598-022-19934-7. Sci Rep. 2022. PMID: 36114353 Free PMC article.
References
-
- Adachi K, Yang Y, Joshi AA, Vasudevan G, Morris A, and McDonald MJ. Consequence of beta 16 and beta 112 replacements on the kinetics of hemoglobin assembly. Biochem Biophys Res Commun 289: 75–79, 2001 - PubMed
-
- Adachi K, Yang Y, Lakka V, Wehrli S, Reddy KS, and Surrey S. Significance of beta116 His (G18) at alpha1beta1 contact sites for alphabeta assembly and autoxidation of hemoglobin. Biochemistry (Mosc) 42: 10252–10259, 2003 - PubMed
-
- Aessopos A, Farmakis D, Karagiorga M, Voskaridou E, Loutradi A, Hatziliami A, Joussef J, Rombos J, and Loukopoulos D. Cardiac involvement in thalassemia intermedia: a multicenter study. Blood 97: 3411–3416, 2001 - PubMed
-
- Alayash AI, Andersen CB, Moestrup SK, and Bulow L. Haptoglobin: the hemoglobin detoxifier in plasma. Trends Biotechnol 31: 2–3, 2013 - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

