systemPipeR: NGS workflow and report generation environment
- PMID: 27650223
- PMCID: PMC5029110
- DOI: 10.1186/s12859-016-1241-0
systemPipeR: NGS workflow and report generation environment
Abstract
Background: Next-generation sequencing (NGS) has revolutionized how research is carried out in many areas of biology and medicine. However, the analysis of NGS data remains a major obstacle to the efficient utilization of the technology, as it requires complex multi-step processing of big data demanding considerable computational expertise from users. While substantial effort has been invested on the development of software dedicated to the individual analysis steps of NGS experiments, insufficient resources are currently available for integrating the individual software components within the widely used R/Bioconductor environment into automated workflows capable of running the analysis of most types of NGS applications from start-to-finish in a time-efficient and reproducible manner.
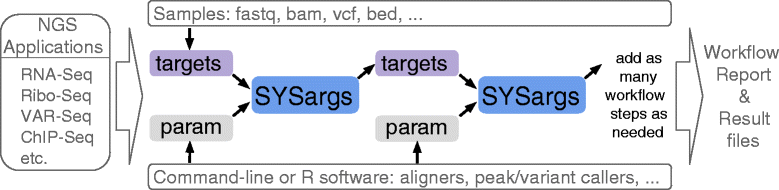
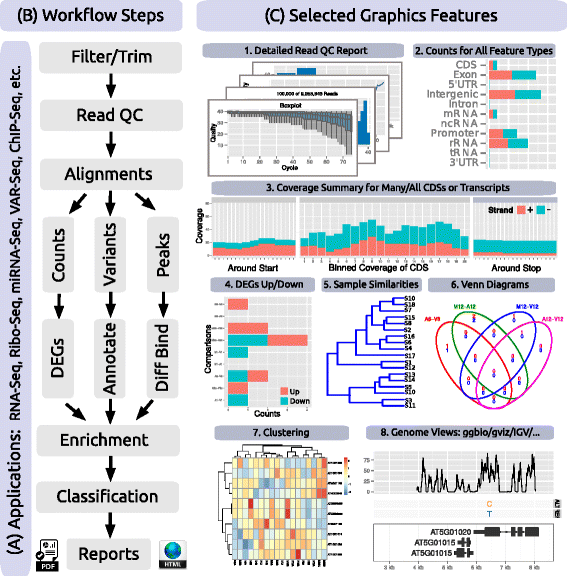
Results: To address this need, we have developed the R/Bioconductor package systemPipeR. It is an extensible environment for both building and running end-to-end analysis workflows with automated report generation for a wide range of NGS applications. Its unique features include a uniform workflow interface across different NGS applications, automated report generation, and support for running both R and command-line software on local computers and computer clusters. A flexible sample annotation infrastructure efficiently handles complex sample sets and experimental designs. To simplify the analysis of widely used NGS applications, the package provides pre-configured workflows and reporting templates for RNA-Seq, ChIP-Seq, VAR-Seq and Ribo-Seq. Additional workflow templates will be provided in the future.
Conclusions: systemPipeR accelerates the extraction of reproducible analysis results from NGS experiments. By combining the capabilities of many R/Bioconductor and command-line tools, it makes efficient use of existing software resources without limiting the user to a set of predefined methods or environments. systemPipeR is freely available for all common operating systems from Bioconductor ( http://bioconductor.org/packages/devel/systemPipeR ).
Keywords: Analysis workflow; ChIP-Seq; Next Generation Sequencing (NGS); RNA-Seq; Ribo-Seq; VAR-Seq.
Figures
References
-
- Lindblad-Toh K, Garber M, Zuk O, Lin MF, Parker BJ, Washietl S, Kheradpour P, Ernst J, Jordan G, Mauceli E, Ward LD, Lowe CB, Holloway AK, Clamp M, Gnerre S, Alföldi J, Beal K, Chang J, Clawson H, Cuff J, Di Palma F, Fitzgerald S, Flicek P, Guttman M, Hubisz MJ, Jaffe DB, Jungreis I, Kent WJ, Kostka D, Lara M, Martins AL, Massingham T, Moltke I, Raney BJ, Rasmussen MD, Robinson J, Stark A, Vilella AJ, Wen J, Xie X, Zody MC, Broad Institute Sequencing Platform and Whole Genome Assembly Team. Baldwin J, Bloom T, Chin CW, Heiman D, Nicol R, Nusbaum C, Young S, Wilkinson J, Worley KC, Kovar CL, Muzny DM, Gibbs RA, Baylor College of Medicine Human Genome Sequencing Center Sequencing Team. Cree A, Dihn HH, Fowler G, Jhangiani S, Joshi V, Lee S, Lewis LR, Nazareth LV, Okwuonu G, Santibanez J, Warren WC, Mardis ER, Weinstock GM, Wilson RK, Genome Institute at Washington University. Delehaunty K, Dooling D, Fronik C, Fulton L, Fulton B, Graves T, Minx P, Sodergren E, Birney E, Margulies EH, Herrero J, Green ED, Haussler D, Siepel A, Goldman N, Pollard KS, Pedersen JS, Lander ES, Kellis M. A high-resolution map of human evolutionary constraint using 29 mammals. Nature. 2011;478(7370):476–82. doi: 10.1038/nature10530. - DOI - PMC - PubMed
-
- Kato-Maeda M, Ho C, Passarelli B, Banaei N, Grinsdale J, Flores L, Anderson J, Murray M, Rose G, Kawamura LM, Pourmand N, Tariq MA, Gagneux S, Hopewell PC. Use of whole genome sequencing to determine the microevolution of Mycobacterium tuberculosis during an outbreak. PLoS ONE. 2013;8(3):58235. doi: 10.1371/journal.pone.0058235. - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

