Effects of high EPA and high DHA fish oils on changes in signaling associated with protein metabolism induced by hindlimb suspension in rats
- PMID: 27650250
- PMCID: PMC5037913
- DOI: 10.14814/phy2.12958
Effects of high EPA and high DHA fish oils on changes in signaling associated with protein metabolism induced by hindlimb suspension in rats
Abstract
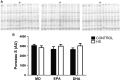
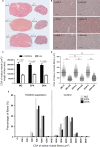
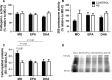
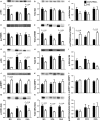
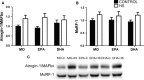
The effects of either eicosapentaenoic (EPA)- or docosahexaenoic (DHA)-rich fish oils on hindlimb suspension (HS)-induced muscle disuse atrophy were compared. Daily oral supplementations (0.3 mL/100 g b.w.) with mineral oil (MO) or high EPA or high DHA fish oils were performed in adult rats. After 2 weeks, the animals were subjected to HS for further 2 weeks. The treatments were maintained alongside HS At the end of 4 weeks, we evaluated: body weight gain, muscle mass and fat depots, composition of fatty acids, cross-sectional areas (CSA) of the soleus muscle and soleus muscle fibers, activities of cathepsin L and 26S proteasome, and content of carbonylated proteins in the soleus muscle. Signaling pathway activities associated with protein synthesis (Akt, p70S6K, S6, 4EBP1, and GSK3-beta) and protein degradation (atrogin-1/MAFbx, and MuRF1) were evaluated. HS decreased muscle mass, CSA of soleus muscle and soleus muscle fibers, and altered signaling associated with protein synthesis (decreased) and protein degradation (increased). The treatment with either fish oil decreased the ratio of omega-6/omega-3 fatty acids and changed protein synthesis-associated signaling. EPA-rich fish oil attenuated the changes induced by HS on 26S proteasome activity, CSA of soleus muscle fibers, and levels of p-Akt, total p70S6K, p-p70S6K/total p70S6K, p-4EBP1, p-GSK3-beta, p-ERK2, and total ERK 1/2 proteins. DHA-rich fish oil attenuated the changes induced by HS on p-4EBP1 and total ERK1 levels. The effects of EPA-rich fish oil on protein synthesis signaling were more pronounced. Both EPA- and DHA-rich fish oils did not impact skeletal muscle mass loss induced by non-inflammatory HS.
Keywords: Hindlimb suspension; muscle atrophy; omega‐3 fatty acids; protein synthesis/degradation.
© 2016 The Authors. Physiological Reports published by Wiley Periodicals, Inc. on behalf of the American Physiological Society and The Physiological Society.
Figures

References
-
- Andrianjafiniony, T. , Dupré‐Aucouturier S., Letexier D., Couchoux H., and Desplanches D.. 2010. Oxidative stress, apoptosis, and proteolysis in skeletal muscle repair after unloading. Am. J. Physiol. Cell Physiol. 299:C307–C315. - PubMed
-
- AOAC . 2005. Official method 996.06. Official methods of analysis of AOAC International, 18th ed. AOAC International, Gaithersburg, MD.
-
- AOCS (2007). Official method Ce 1j‐07. Official methods and recommended practices of the AOCS. 6th ed AOCS, Champaign, IL, USA.
-
- Baillie, R. A. , Takada R., Nakamura M., and Clarke S. D.. 1999. Coordinate induction of peroxisomal acyl‐CoA oxidase and UCP‐3 by dietary fish oil: a mechanism for decreased body fat deposition. Prostaglandins Leukot. Essent. Fatty Acids 60:351–356. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials
Miscellaneous

